Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington's disease mice
- PMID: 25526803
- PMCID: PMC4297405
- DOI: 10.1186/s40478-014-0178-7
Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington's disease mice
Abstract
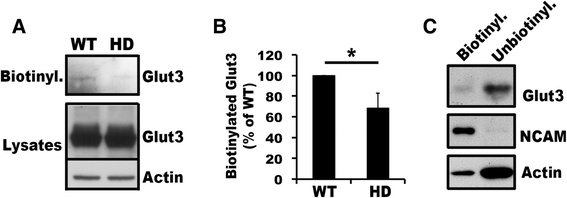
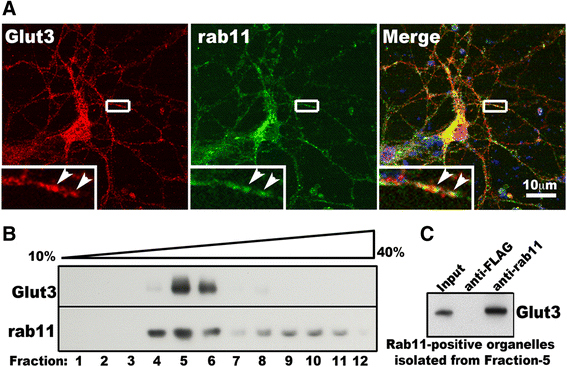
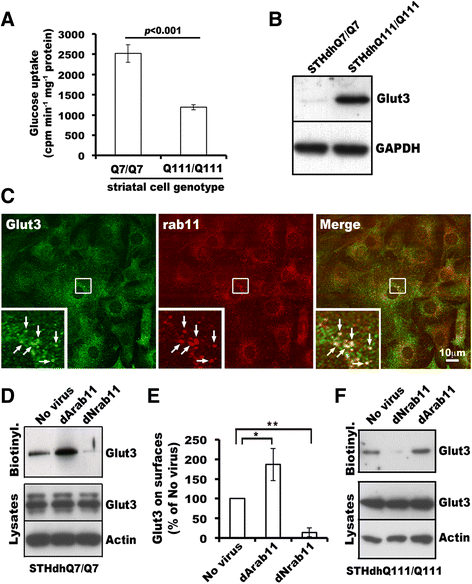
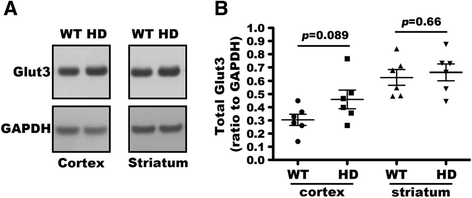
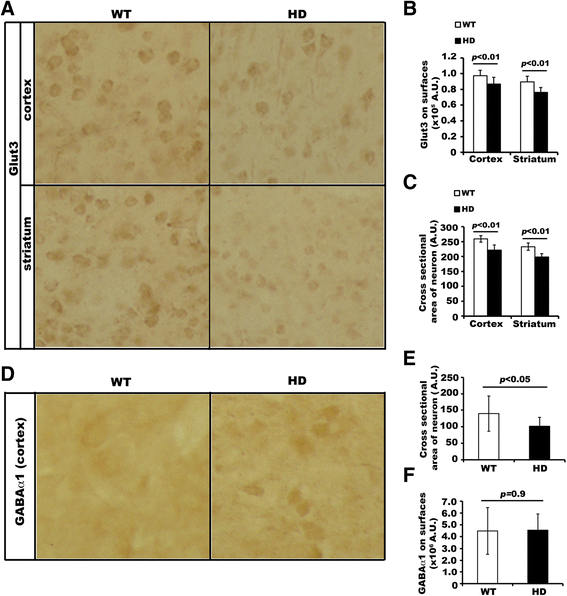
Huntington's disease (HD) disturbs glucose metabolism in the brain by poorly understood mechanisms. HD neurons have defective glucose uptake, which is attenuated upon enhancing rab11 activity. Rab11 regulates numerous receptors and transporters trafficking onto cell surfaces; its diminished activity in HD cells affects the recycling of transferrin receptor and neuronal glutamate/cysteine transporter EAAC1. Glucose transporter 3 (Glut3) handles most glucose uptake in neurons. Here we investigated rab11 involvement in Glut3 trafficking. Glut3 was localized to rab11 positive puncta in primary neurons and immortalized striatal cells by immunofluorescence labeling and detected in rab11-enriched endosomes immuno-isolated from mouse brain by Western blot. Expression of dominant active and negative rab11 mutants in clonal striatal cells altered the levels of cell surface Glut3 suggesting a regulation by rab11. About 4% of total Glut3 occurred at the cell surface of primary WT neurons. HD(140Q/140Q) neurons had significantly less cell surface Glut3 than did WT neurons. Western blot analysis revealed comparable levels of Glut3 in the striatum and cortex of WT and HD(140Q/140Q) mice. However, brain slices immunolabeled with an antibody recognizing an extracellular epitope to Glut3 showed reduced surface expression of Glut3 in the striatum and cortex of HD(140Q/140Q) mice compared to that of WT mice. Surface labeling of GABAα1 receptor, which is not dependent on rab11, was not different between WT and HD(140Q/140Q) mouse brain slices. These data define Glut3 to be a rab11-dependent trafficking cargo and suggest that impaired Glut3 trafficking arising from rab11 dysfunction underlies the glucose hypometabolism observed in HD.
Figures
Similar articles
-
Aberrant Rab11-dependent trafficking of the neuronal glutamate transporter EAAC1 causes oxidative stress and cell death in Huntington's disease.J Neurosci. 2010 Mar 31;30(13):4552-61. doi: 10.1523/JNEUROSCI.5865-09.2010. J Neurosci. 2010. PMID: 20357106 Free PMC article.
-
Deficient Rab11 activity underlies glucose hypometabolism in primary neurons of Huntington's disease mice.Biochem Biophys Res Commun. 2012 May 18;421(4):727-30. doi: 10.1016/j.bbrc.2012.04.070. Epub 2012 Apr 20. Biochem Biophys Res Commun. 2012. PMID: 22542623
-
Disruption of Rab11 activity in a knock-in mouse model of Huntington's disease.Neurobiol Dis. 2009 Nov;36(2):374-83. doi: 10.1016/j.nbd.2009.08.003. Epub 2009 Aug 20. Neurobiol Dis. 2009. PMID: 19699304 Free PMC article.
-
Nature and cause of mitochondrial dysfunction in Huntington's disease: focusing on huntingtin and the striatum.J Neurochem. 2010 Jul;114(1):1-12. doi: 10.1111/j.1471-4159.2010.06741.x. Epub 2010 Apr 9. J Neurochem. 2010. PMID: 20403078 Review.
-
Orchestration of cell surface proteins by Rab11.Trends Cell Biol. 2014 Jul;24(7):407-15. doi: 10.1016/j.tcb.2014.02.004. Epub 2014 Mar 24. Trends Cell Biol. 2014. PMID: 24675420 Review.
Cited by
-
Rab11 and Its Role in Neurodegenerative Diseases.ASN Neuro. 2022 Jan-Dec;14:17590914221142360. doi: 10.1177/17590914221142360. ASN Neuro. 2022. PMID: 36464817 Free PMC article. Review.
-
GLUT3 promotes macrophage signaling and function via RAS-mediated endocytosis in atopic dermatitis and wound healing.J Clin Invest. 2023 Nov 1;133(21):e170706. doi: 10.1172/JCI170706. J Clin Invest. 2023. PMID: 37721853 Free PMC article.
-
AMPK role in epilepsy: a promising therapeutic target?J Neurol. 2024 Feb;271(2):748-771. doi: 10.1007/s00415-023-12062-w. Epub 2023 Nov 27. J Neurol. 2024. PMID: 38010498 Review.
-
Glucose Metabolic Dysfunction in Neurodegenerative Diseases-New Mechanistic Insights and the Potential of Hypoxia as a Prospective Therapy Targeting Metabolic Reprogramming.Int J Mol Sci. 2021 May 31;22(11):5887. doi: 10.3390/ijms22115887. Int J Mol Sci. 2021. PMID: 34072616 Free PMC article. Review.
-
Single-nuclei transcriptome analysis of Huntington disease iPSC and mouse astrocytes implicates maturation and functional deficits.iScience. 2022 Dec 6;26(1):105732. doi: 10.1016/j.isci.2022.105732. eCollection 2023 Jan 20. iScience. 2022. PMID: 36590162 Free PMC article.
References
-
- Young AB, Penney JB, Starosta-Rubinstein S, Markel DS, Berent S, Giordani B, Ehrenkaufer R, Jewett D, Hichwa R. PET scan investigations of Huntington's disease: cerebral metabolic correlates of neurological features and functional decline. Ann Neurol. 1986;20(3):296–303. doi: 10.1002/ana.410200305. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

