Interest of preoperative immunonutrition in liver resection for cancer: study protocol of the PROPILS trial, a multicenter randomized controlled phase IV trial
- PMID: 25523036
- PMCID: PMC4302113
- DOI: 10.1186/1471-2407-14-980
Interest of preoperative immunonutrition in liver resection for cancer: study protocol of the PROPILS trial, a multicenter randomized controlled phase IV trial
Abstract
Background: Malnutrition is an independent risk factor of postoperative morbidity and mortality and it's observed in 20 to 50% of surgical patients. Preoperative interventions to optimize the nutritional status, reduce postoperative complications and enteral nutrition has proven to be superior to the parenteral one. Moreover, regardless of the nutritional status of the patient, surgery impairs the immunological response, thus increasing the risk of postoperative sepsis. Immunonutrition has been developed to improve the immunometabolic host response in perioperative period and it has been proven to reduce significantly postoperative infectious complications and length of hospital stay in patients undergoing elective gastrointestinal surgery for tumors. We hypothesize that a preoperative oral immunonutrition (ORAL IMPACT®) can reduce postoperative morbidity in liver resection for cancer.
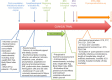
Methods/design: Prospective multicenter randomized placebo-controlled double-blind phase IV trial with two parallel treatment groups receiving either study product (ORAL IMPACT®) or control supplement (isocaloric isonitrogenous supplement--IMPACT CONTROL®) for 7 days before liver resection for cancer. A total of 400 patients will be enrolled. Patients will be stratified according to the type of hepatectomy, the presence of chronic liver disease and the investigator center. The main end-point is to evaluate in intention-to-treat analysis the overall 30-day morbidity. Secondary end-points are to assess the 30-day infectious and non-infectious morbidity, length of antibiotic treatment and hospital stay, modifications on total food intake, compliance to treatment, side-effects of immunonutrition, impact on liver regeneration and sarcopenia, and to perform a medico-economic analysis.
Discussion: The overall morbidity rate after liver resection is 22% to 42%. Infectious post-operative complications (12% to 23%) increase the length of hospital stay and costs and are responsible for a quarter of 30-day mortality. Various methods have been advocated to decrease the rate of postoperative complications but there is no evidence to support or refute the use of any treatment and further trials are required. The effects of preoperative oral immunonutrition in non-cirrhotic patients undergoing liver resection for cancer are unknown. The present trial is designed to evaluate whether the administration of a short-term preoperative oral immunonutrition can reduce postoperative morbidity in non-cirrhotic patients undergoing liver resection for cancer.
Trial registration: Clinicaltrial.gov: NCT02041871.
Figures
Similar articles
-
The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: a prospective, randomized, clinical trial.Ann Surg. 2008 Aug;248(2):212-20. doi: 10.1097/SLA.0b013e318180a3c1. Ann Surg. 2008. PMID: 18650630 Clinical Trial.
-
Multicentre factorial randomized clinical trial of perioperative immunonutrition versus standard nutrition for patients undergoing surgical resection of oesophageal cancer.Br J Surg. 2018 Sep;105(10):1262-1272. doi: 10.1002/bjs.10923. Epub 2018 Jul 12. Br J Surg. 2018. PMID: 29999517 Clinical Trial.
-
Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer.Br J Surg. 2012 May;99(5):621-9. doi: 10.1002/bjs.8706. Epub 2012 Feb 24. Br J Surg. 2012. PMID: 22367794 Clinical Trial.
-
Immunonutrition in patients undergoing esophageal cancer resection.Dis Esophagus. 2011 Apr;24(3):160-5. doi: 10.1111/j.1442-2050.2010.01117.x. Epub 2010 Oct 13. Dis Esophagus. 2011. PMID: 20946133 Review.
-
Preoperative immunonutrition: cost-benefit analysis.JPEN J Parenter Enteral Nutr. 2005 Jan-Feb;29(1 Suppl):S57-61. doi: 10.1177/01486071050290S1S57. JPEN J Parenter Enteral Nutr. 2005. PMID: 15709546 Review.
Cited by
-
Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations.World J Surg. 2016 Oct;40(10):2425-40. doi: 10.1007/s00268-016-3700-1. World J Surg. 2016. PMID: 27549599 Review.
-
Effect of Qihuang Decoction Combined with Enteral Nutrition on Postoperative Gastric Cancer of Nutrition and Immune Function.Evid Based Complement Alternat Med. 2020 Feb 29;2020:1795107. doi: 10.1155/2020/1795107. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32215032 Free PMC article.
-
The Role of Immunonutrition in Patients Undergoing Pancreaticoduodenectomy.Nutrients. 2020 Aug 23;12(9):2547. doi: 10.3390/nu12092547. Nutrients. 2020. PMID: 32842475 Free PMC article. Review.
-
GNRs@SiO₂-FA in combination with radiotherapy induces the apoptosis of HepG2 cells by modulating the expression of apoptosis-related proteins.Int J Mol Med. 2015 Nov;36(5):1282-90. doi: 10.3892/ijmm.2015.2358. Epub 2015 Sep 30. Int J Mol Med. 2015. PMID: 26648274 Free PMC article.
-
Is preoperative protein-rich nutrition effective on postoperative outcome in non-small cell lung cancer surgery? A prospective randomized study.J Cardiothorac Surg. 2016 Jan 19;11:14. doi: 10.1186/s13019-016-0407-1. J Cardiothorac Surg. 2016. PMID: 26782276 Free PMC article. Clinical Trial.
References
-
- Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/00000658-200210000-00001. - DOI - PMC - PubMed
-
- Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, Jaeck D, Saric J, Le Treut YP, Belghiti J, Mantion G, Mentha G. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg. 2006;244:524–535. doi: 10.1097/01.sla.0000246847.02058.1b. - DOI - PMC - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/980/prepub
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical