Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: immunological properties
- PMID: 25520151
- PMCID: PMC4396058
- DOI: 10.1186/s12866-014-0233-3
Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: immunological properties
Abstract
Background: Currently, a licensed vaccine for Dengue Virus (DENV) is not yet available. Virus-like particles (VLP) have shown considerable promise for use as vaccines and have many advantages compared to many other types of viral vaccines. VLPs have been found to have high immunogenic potencies, providing protection against various pathogens.
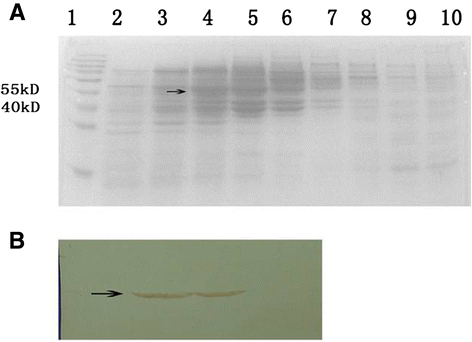
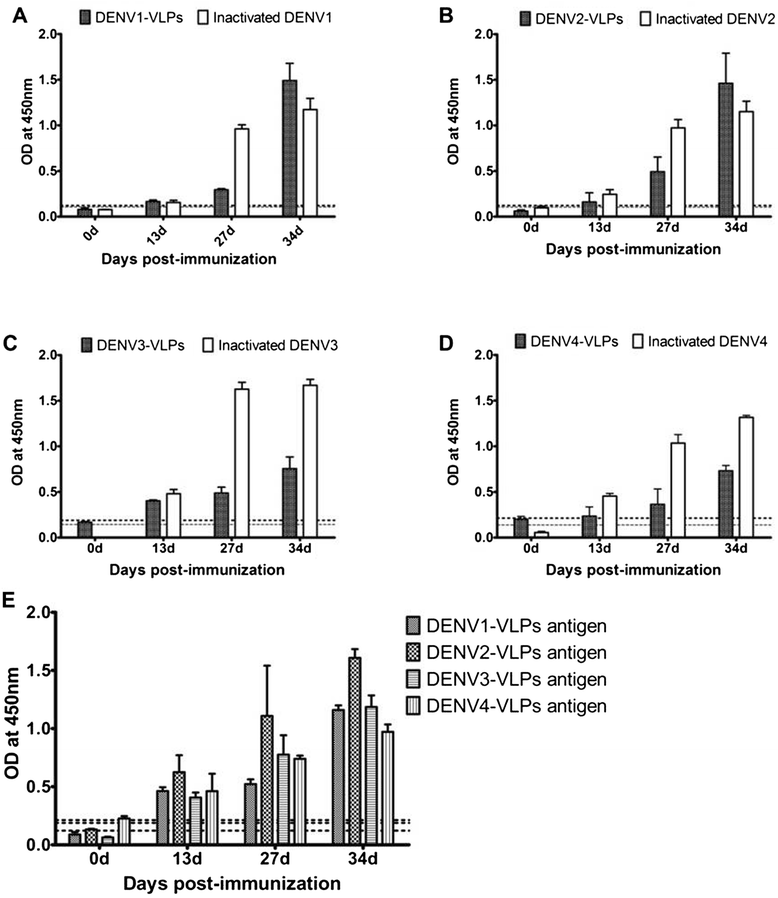
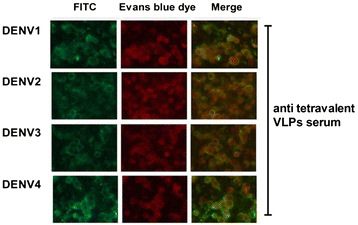
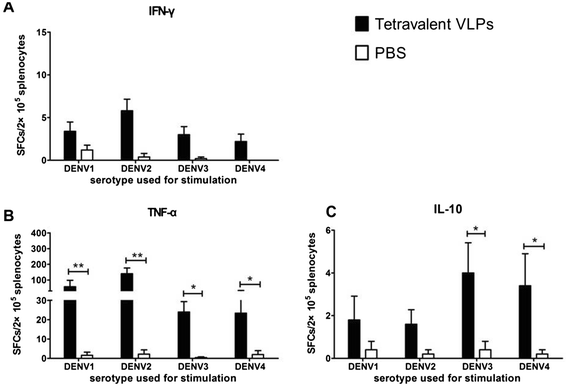
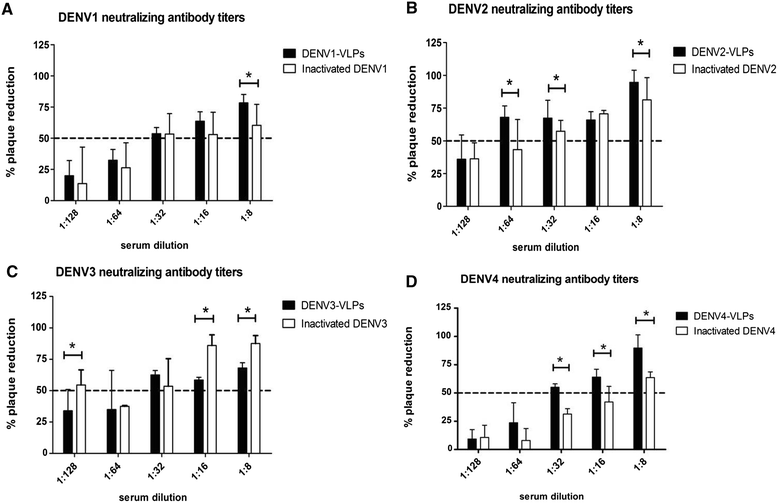
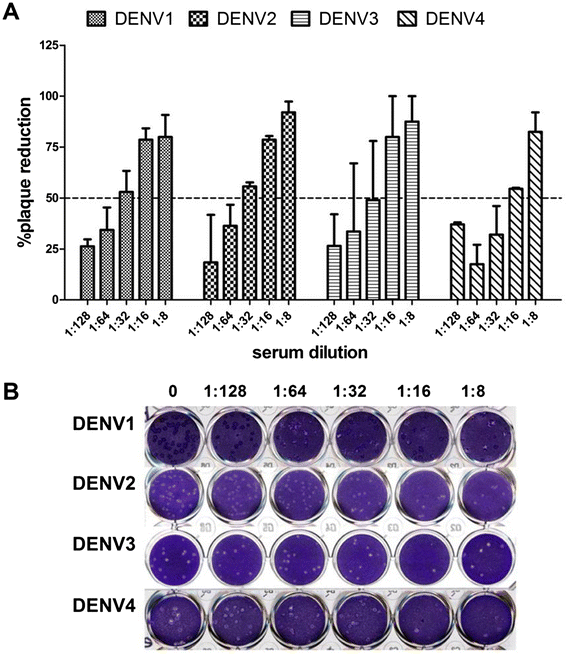
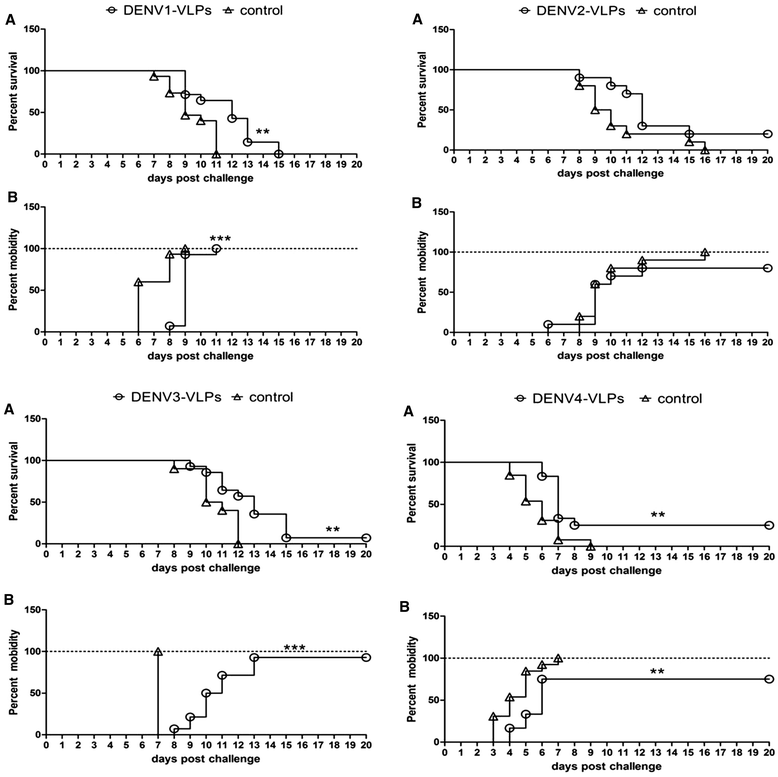
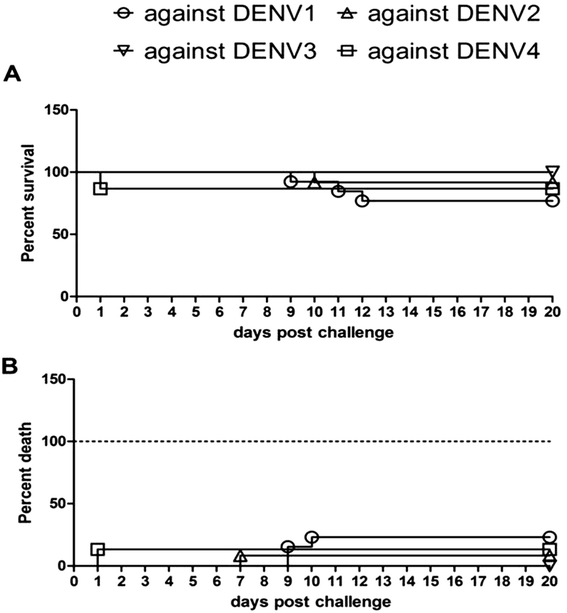
Results: In the current study, four DENV-VLP serotypes were successfully expressed in Pichia pastoris, based on co-expression of the prM and E proteins. The effects of a tetravalent VLP vaccine were also examined. Immunization with purified, recombinant, tetravalent DENV1-4 VLPs induced specific antibodies against all DENV1-4 antigens in mice. The antibody titers were higher after immunization with the tetravalent VLP vaccine compared to titers after immunization with any of the dengue serotype VLPs alone. Indirect immunofluorescence assay (IFA) results indicated that sera from VLP immunized mice recognized the native viral antigens. TNF-α and IL-10 were significantly higher in mice immunized with tetravalent DENV-VLP compared to those mice received PBS. The tetravalent VLP appeared to stimulate neutralizing antibodies against each viral serotype, as shown by PRNT50 analysis (1:32 against DENV1 and 2, and 1:16 against DENV3 and 4). The highest titers with the tetravalent VLP vaccine were still a little lower than the monovalent VLP against the corresponding serotype. The protection rates of tetravalent DENV-VLP immune sera against challenges with DENV1 to 4 serotypes in suckling mice were 77, 92, 100, and 100%, respectively, indicating greater protective efficacy compared with monovalent immune sera.
Conclusions: Our results provide an important basis for the development of the dengue VLP as a promising non-infectious candidate vaccine for dengue infection.
Figures
Similar articles
-
An Envelope-Modified Tetravalent Dengue Virus-Like-Particle Vaccine Has Implications for Flavivirus Vaccine Design.J Virol. 2017 Nov 14;91(23):e01181-17. doi: 10.1128/JVI.01181-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956764 Free PMC article.
-
Virus-like particles displaying envelope domain III of dengue virus type 2 induce virus-specific antibody response in mice.Vaccine. 2013 Jan 30;31(6):873-8. doi: 10.1016/j.vaccine.2012.12.016. Epub 2012 Dec 20. Vaccine. 2013. PMID: 23261049
-
Tests in mice of a dengue vaccine candidate made of chimeric Junin virus-like particles and conserved dengue virus envelope sequences.Appl Microbiol Biotechnol. 2016 Jan;100(1):125-33. doi: 10.1007/s00253-015-6973-7. Epub 2015 Sep 19. Appl Microbiol Biotechnol. 2016. PMID: 26386688
-
A recombinant, chimeric tetravalent dengue vaccine candidate based on a dengue virus serotype 2 backbone.Expert Rev Vaccines. 2016;15(4):497-508. doi: 10.1586/14760584.2016.1128328. Epub 2016 Feb 22. Expert Rev Vaccines. 2016. PMID: 26635182 Review.
-
Development of a recombinant, chimeric tetravalent dengue vaccine candidate.Vaccine. 2015 Dec 10;33(50):7112-20. doi: 10.1016/j.vaccine.2015.11.022. Epub 2015 Nov 14. Vaccine. 2015. PMID: 26585500 Review.
Cited by
-
Mosquito Cell-Derived Japanese Encephalitis Virus-Like Particles Induce Specific Humoral and Cellular Immune Responses in Mice.Viruses. 2020 Mar 19;12(3):336. doi: 10.3390/v12030336. Viruses. 2020. PMID: 32204533 Free PMC article.
-
Development of Peptide Vaccines in Dengue.Curr Pharm Des. 2018;24(11):1157-1173. doi: 10.2174/1381612823666170913163904. Curr Pharm Des. 2018. PMID: 28914200 Free PMC article. Review.
-
Yeast-Based Virus-like Particles as an Emerging Platform for Vaccine Development and Delivery.Vaccines (Basel). 2023 Feb 18;11(2):479. doi: 10.3390/vaccines11020479. Vaccines (Basel). 2023. PMID: 36851356 Free PMC article. Review.
-
Production and Purification of Dengue Virus-like Particles from COS-1 Cells.Bio Protoc. 2019 Jun 20;9(12):e3280. doi: 10.21769/BioProtoc.3280. eCollection 2019 Jun 20. Bio Protoc. 2019. PMID: 33654796 Free PMC article.
-
Virus-Like Particle Systems for Vaccine Development against Viruses in the Flaviviridae Family.Vaccines (Basel). 2019 Sep 20;7(4):123. doi: 10.3390/vaccines7040123. Vaccines (Basel). 2019. PMID: 31547131 Free PMC article. Review.
References
-
- Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachu-eke S, Kiatpolpoj S, Innis BL, Rothman AL, Nisalak A, Ennis FA. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. - DOI - PubMed
-
- Kuo CH, Tai DI, Chang-Chien CS, Lan CK, Chiou SS, Liaw YF. Liver biochemical tests and dengue fever. Am J Trop Med Hyg. 1992;47:265–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

