Modeling options to manage type 1 wild poliovirus imported into Israel in 2013
- PMID: 25505296
- PMCID: PMC7887763
- DOI: 10.1093/infdis/jiu674
Modeling options to manage type 1 wild poliovirus imported into Israel in 2013
Abstract
Background: After 25 years without poliomyelitis cases caused by circulating wild poliovirus (WPV) in Israel, sewage sampling detected WPV type 1 (WPV1) in April 2013, despite high vaccination coverage with only inactivated poliovirus vaccine (IPV) since 2005.
Methods: We used a differential equation-based model to simulate the dynamics of poliovirus transmission and population immunity in Israel due to past exposure to WPV and use of oral poliovirus vaccine (OPV) in addition to IPV. We explored the influences of various immunization options to stop imported WPV1 circulation in Israel.
Results: We successfully modeled the potential for WPVs to circulate without detected cases in Israel. Maintaining a sequential IPV/OPV schedule instead of switching to an IPV-only schedule in 2005 would have kept population immunity high enough in Israel to prevent WPV1 circulation. The Israeli response to WPV1 detection prevented paralytic cases; a more rapid response might have interrupted transmission more quickly.
Conclusions: IPV-based protection alone might not provide sufficient population immunity to prevent poliovirus transmission after an importation. As countries transition to IPV in immunization schedules, they may need to actively manage population immunity and consider continued use of OPV, to avoid the potential circulation of imported live polioviruses before globally coordinated cessation of OPV use.
Keywords: IPV; OPV; eradication; polio; population immunity; vaccine.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Figures
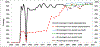

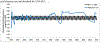
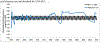
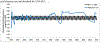
Similar articles
-
Trade-offs of different poliovirus vaccine options for outbreak response in the United States and other countries that only use inactivated poliovirus vaccine (IPV) in routine immunization.Vaccine. 2024 Feb 6;42(4):819-827. doi: 10.1016/j.vaccine.2023.12.081. Epub 2024 Jan 12. Vaccine. 2024. PMID: 38218668 Free PMC article. Review.
-
Managing population immunity to reduce or eliminate the risks of circulation following the importation of polioviruses.Vaccine. 2015 Mar 24;33(13):1568-77. doi: 10.1016/j.vaccine.2015.02.013. Epub 2015 Feb 18. Vaccine. 2015. PMID: 25701673 Free PMC article.
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 5;12(12):CD011260. doi: 10.1002/14651858.CD011260.pub2. Cochrane Database Syst Rev. 2019. PMID: 31801180 Free PMC article.
-
Modeling strategies to increase population immunity and prevent poliovirus transmission in 2 high-risk areas in northern India.J Infect Dis. 2014 Nov 1;210 Suppl 1:S398-411. doi: 10.1093/infdis/jit844. J Infect Dis. 2014. PMID: 25316861
-
Poliovirus vaccines. Progress toward global poliomyelitis eradication and changing routine immunization recommendations in the United States.Pediatr Clin North Am. 2000 Apr;47(2):287-308. doi: 10.1016/s0031-3955(05)70208-x. Pediatr Clin North Am. 2000. PMID: 10761505 Review.
Cited by
-
Trade-offs of different poliovirus vaccine options for outbreak response in the United States and other countries that only use inactivated poliovirus vaccine (IPV) in routine immunization.Vaccine. 2024 Feb 6;42(4):819-827. doi: 10.1016/j.vaccine.2023.12.081. Epub 2024 Jan 12. Vaccine. 2024. PMID: 38218668 Free PMC article. Review.
-
Modeling undetected poliovirus circulation following the 2022 outbreak in the United States.Expert Rev Vaccines. 2024 Jan-Dec;23(1):186-195. doi: 10.1080/14760584.2023.2299401. Epub 2024 Jan 4. Expert Rev Vaccines. 2024. PMID: 38164695 Free PMC article.
-
Modeling Poliovirus Transmission and Responses in New York State.J Infect Dis. 2024 Apr 12;229(4):1097-1106. doi: 10.1093/infdis/jiad355. J Infect Dis. 2024. PMID: 37596838 Free PMC article.
-
The role of a genetically stable, novel oral type 2 poliovirus vaccine in the poliomyelitis endgame.Rev Panam Salud Publica. 2023 Jul 3;47:e99. doi: 10.26633/RPSP.2023.99. eCollection 2023. Rev Panam Salud Publica. 2023. PMID: 37405121 Free PMC article.
-
Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences.Risk Anal. 2024 Feb;44(2):366-378. doi: 10.1111/risa.14158. Epub 2023 Jun 21. Risk Anal. 2024. PMID: 37344934 Free PMC article. Review.
References
-
- Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, Modlin JF, Patriarca PA, Sutter RW, Wright PF, Wassilak SGF, Cochi SL, Kim J-H, Thompson KM. Review and assessment of poliovirus immunity and transmission: Synthesis of knowledge gaps and identification of research needs. Risk Anal 2013; 33(4):606–646. - PMC - PubMed
-
- Goldblum N, Swartz TA. The Israeli experience in the control of poliomyelitis during a quarter af a century, 1957-1982. Rev Infect Dis 1984; 6 (2):S313–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

