Antibody-incompatible kidney transplantation in 2015 and beyond
- PMID: 25500804
- PMCID: PMC4832984
- DOI: 10.1093/ndt/gfu375
Antibody-incompatible kidney transplantation in 2015 and beyond
Abstract
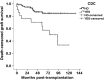
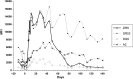
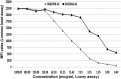
Rejection caused by donor-specific antibodies (principally ABO and HLA antibodies) has become one of the major barriers to successful long-term transplantation. This review focuses on clinical outcomes in antibody-incompatible transplantation, the current state of the science underpinning clinical observations, and how these may be translated into further novel therapies. The clinical outcomes for allografts facing donor-specific antibodies are at present determined largely by the use of agents developed in the 20th century for the treatment of T-lymphocyte-mediated cellular rejection, such as interleukin-2 agents and anti-thymocyte globulin. These treatments are partially effective, because acute antibody-mediated rejection is mediated to a considerable extent by T lymphocytes. However these treatments are essentially ineffective in chronic antibody-mediated rejection. Future therapies for the prevention and treatment of antibody-mediated rejection are likely to fall into the categories of those that reduce antibody production, extracorporeal antibody removal and disruption of the effector arms of antibody-mediated tissue damage.
Keywords: ABO; HLA; antibody; antibody-mediated rejection; kidney transplant.
© The Author 2014. Published by Oxford University Press on behalf of ERA-EDTA. All rights reserved.
Figures
Similar articles
-
Neither pre-transplant rituximab nor splenectomy affects de novo HLA antibody production after renal transplantation.Kidney Int. 2014 Feb;85(2):425-30. doi: 10.1038/ki.2013.291. Epub 2013 Aug 14. Kidney Int. 2014. PMID: 23945498
-
Antibody-mediated rejection of an HLA-identical, ABO-incompatible kidney transplant after two failed cadaver transplants.Transplantation. 1994 Sep 27;58(6):723-5. Transplantation. 1994. PMID: 7940694 No abstract available.
-
Efficacy and safety of tandem hemodialysis and immunoadsorption to desensitize kidney transplant candidates.Exp Clin Transplant. 2015 Apr;13 Suppl 1:165-9. Exp Clin Transplant. 2015. PMID: 25894148
-
Therapeutic apheresis in renal transplantation; current practices.J Clin Apher. 2014 Aug;29(4):206-10. doi: 10.1002/jca.21330. Epub 2014 May 27. J Clin Apher. 2014. PMID: 24863952 Review.
-
New choices for patients needing kidney transplantation across antibody barriers.J Ren Care. 2008 Jun;34(2):85-93. doi: 10.1111/j.1755-6686.2008.00025.x. J Ren Care. 2008. PMID: 18498573 Review.
Cited by
-
HLA Antibody Incompatible Renal Transplantation: Long-term Outcomes Similar to Deceased Donor Transplantation.Transplant Direct. 2021 Jul 19;7(8):e732. doi: 10.1097/TXD.0000000000001183. eCollection 2021 Aug. Transplant Direct. 2021. PMID: 34291154 Free PMC article.
-
Individualized Preconditioning for ABO-Incompatible Living-Donor Kidney Transplantation: An Initial Report of 48 Cases from China.Ann Transplant. 2020 Feb 7;25:e920224. doi: 10.12659/AOT.920224. Ann Transplant. 2020. PMID: 32029699 Free PMC article.
-
Living donor kidney transplantation after desensitization in cross-match positive high sensitized patients.Hippokratia. 2020 Oct-Dec;24(4):182-190. Hippokratia. 2020. PMID: 35023894 Free PMC article.
-
Pregnancy-Induced Sensitization Promotes Sex Disparity in Living Donor Kidney Transplantation.J Am Soc Nephrol. 2017 Oct;28(10):3025-3033. doi: 10.1681/ASN.2016101059. Epub 2017 May 8. J Am Soc Nephrol. 2017. PMID: 28483798 Free PMC article.
-
Analysis of Factors Influencing Kidney Function of Recipients After Renal Transplantation in Southwestern China: A Retrospective Study.Front Med (Lausanne). 2020 Nov 12;7:519582. doi: 10.3389/fmed.2020.519582. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33282882 Free PMC article.
References
-
- Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. New Engl J Med 1969; 280: 735–739 - PubMed
-
- Higgins R, Lowe D, Hathaway M, et al. HLA antibody incompatible renal transplantation: excellent medium term outcomes with negative cytotoxic crossmatch. Transplantation 2011; 92; 900–906 - PubMed
-
- Gupta A, Iveson V, Varagunam M, et al. Pretransplant donor-specific antibodies in cytotoxic negative crossmatch kidney transplants: are they relevant? Transplantation 2008; 85: 1200–1204 - PubMed
-
- Singh N, Djamali A, Lorentzen D, et al. Pretransplant donor-specific antibodies detected by single-antigen bead flow cytometry are associated with inferior kidney transplant outcomes. Transplantation 2010; 90: 1079–1084 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

