Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis
- PMID: 25496490
- PMCID: PMC4273482
- DOI: 10.1186/s12931-014-0157-3
Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease with poor prognosis. The kinase inhibitor nintedanib specific for vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR) and fibroblast growth factor receptor (FGFR) significantly reduced the rate of decline of forced vital capacity versus placebo.
Aim: To determine the in vitro effect of nintedanib on primary human lung fibroblasts.
Methods: Fibroblasts were isolated from lungs of IPF patients and from non-fibrotic controls. We assessed the effect of VEGF, PDGF-BB and basic FGF (bFGF) ± nintedanib on: (i) expression/activation of VEGFR, PDGFR, and FGFR, (ii) cell proliferation, secretion of (iii) matrix metalloproteinases (MMP), (iv) tissue inhibitor of metalloproteinase (TIMP), and (v) collagen.
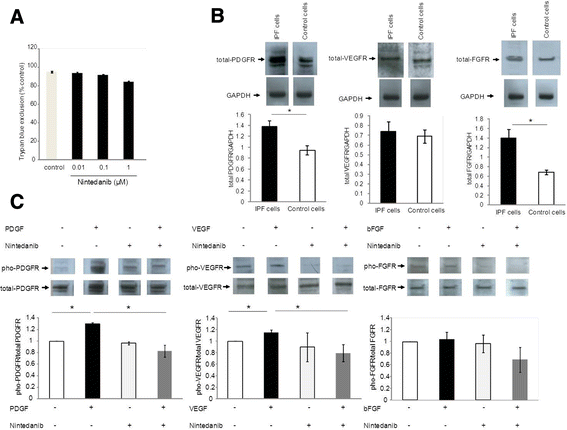
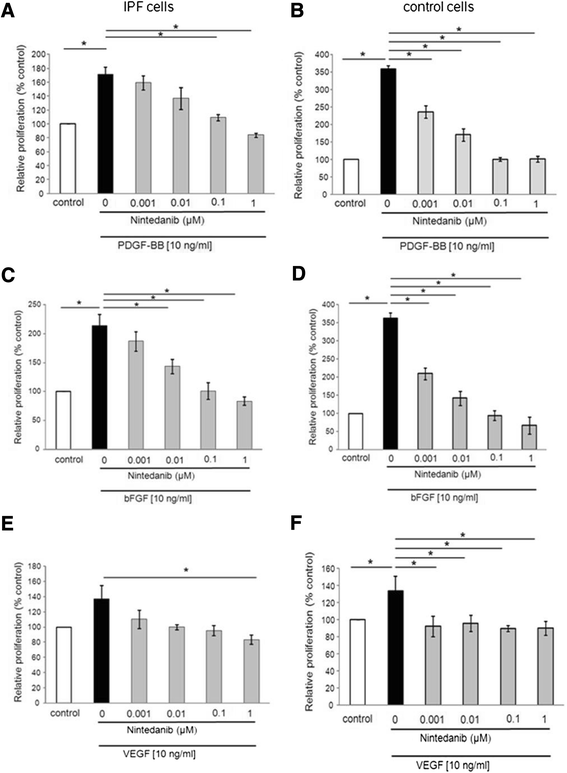
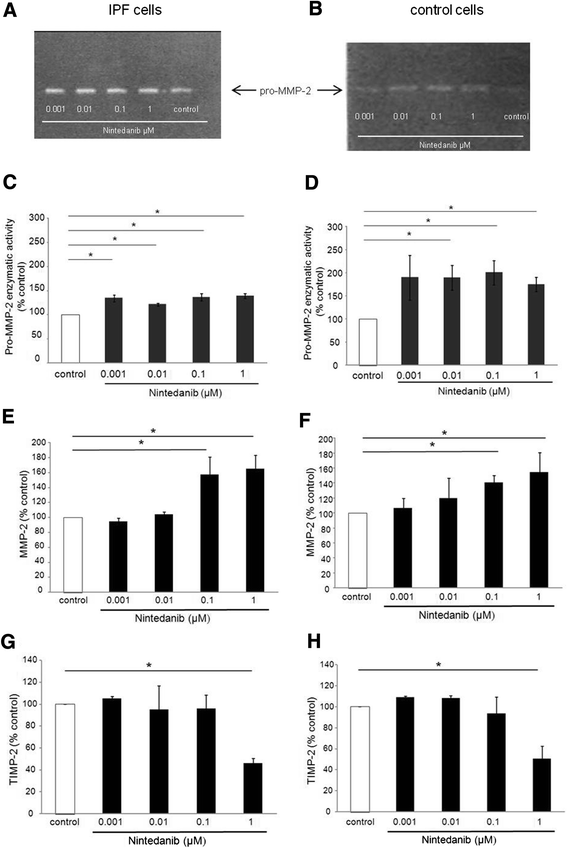
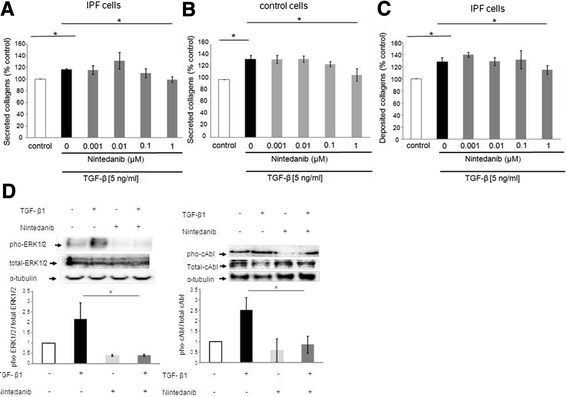
Results: IPF fibroblasts expressed higher levels of PDGFR and FGFR than controls. PDGF-BB, bFGF, and VEGF caused a pro-proliferative effect which was prevented by nintedanib. Nintedanib enhanced the expression of pro-MMP-2, and inhibited the expression of TIMP-2. Transforming growth factor-beta-induced secretion of collagens was inhibited by nintedanib.
Conclusion: Our data demonstrate a significant anti-fibrotic effect of nintedanib in IPF fibroblasts. This effect consists of the drug's anti-proliferative capacity, and on its effect on the extracellular matrix, the degradation of which seems to be enhanced.
Figures
Similar articles
-
Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis.J Pharmacol Exp Ther. 2014 May;349(2):209-20. doi: 10.1124/jpet.113.208223. Epub 2014 Feb 20. J Pharmacol Exp Ther. 2014. PMID: 24556663
-
Fibroblast-matrix interplay: Nintedanib and pirfenidone modulate the effect of IPF fibroblast-conditioned matrix on normal fibroblast phenotype.Respirology. 2018 Aug;23(8):756-763. doi: 10.1111/resp.13287. Epub 2018 Mar 12. Respirology. 2018. PMID: 29532550
-
Nintedanib modulates surfactant protein-D expression in A549 human lung epithelial cells via the c-Jun N-terminal kinase-activator protein-1 pathway.Pulm Pharmacol Ther. 2015 Jun;32:29-36. doi: 10.1016/j.pupt.2015.03.001. Epub 2015 Apr 2. Pulm Pharmacol Ther. 2015. PMID: 25843005
-
Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis.Eur Respir J. 2015 May;45(5):1434-45. doi: 10.1183/09031936.00174914. Epub 2015 Mar 5. Eur Respir J. 2015. PMID: 25745043 Free PMC article. Review.
-
The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis.Eur Respir J. 2015 May;45(5):1426-33. doi: 10.1183/09031936.00149614. Epub 2015 Mar 5. Eur Respir J. 2015. PMID: 25745048 Review.
Cited by
-
Therapeutic potential of targeting kinase inhibition in patients with idiopathic pulmonary fibrosis.Yeungnam Univ J Med. 2020 Oct;37(4):269-276. doi: 10.12701/yujm.2020.00458. Epub 2020 Jul 22. Yeungnam Univ J Med. 2020. PMID: 32693446 Free PMC article.
-
Pharmacological Treatments and Therapeutic Targets in Muscle Dystrophies Generated by Alterations in Dystrophin-Associated Proteins.Medicina (Kaunas). 2024 Jun 27;60(7):1060. doi: 10.3390/medicina60071060. Medicina (Kaunas). 2024. PMID: 39064489 Free PMC article. Review.
-
Subtypes identification on heart failure with preserved ejection fraction via network enhancement fusion using multi-omics data.Comput Struct Biotechnol J. 2021 Mar 17;19:1567-1578. doi: 10.1016/j.csbj.2021.03.010. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 33868594 Free PMC article.
-
Idiopathic Pulmonary Fibrosis: Pathogenesis and the Emerging Role of Long Non-Coding RNAs.Int J Mol Sci. 2020 Jan 14;21(2):524. doi: 10.3390/ijms21020524. Int J Mol Sci. 2020. PMID: 31947693 Free PMC article. Review.
-
Combination of losartan with pirfenidone: a protective anti-fibrotic against pulmonary fibrosis induced by bleomycin in rats.Sci Rep. 2024 Apr 16;14(1):8729. doi: 10.1038/s41598-024-59395-8. Sci Rep. 2024. PMID: 38622264 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

