Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings
- PMID: 25491299
- PMCID: PMC4288143
- DOI: 10.1167/iovs.14-15415
Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings
Abstract
Purpose: Because human bone marrow (BM) CD34+ stem cells home into damaged tissue and may play an important role in tissue repair, this pilot clinical trial explored the safety and feasibility of intravitreal autologous CD34+ BM cells as potential therapy for ischemic or degenerative retinal conditions.
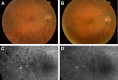
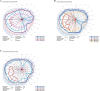
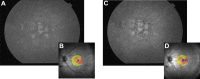
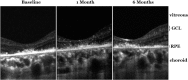
Methods: This prospective study enrolled six subjects (six eyes) with irreversible vision loss from retinal vascular occlusion, hereditary or nonexudative age-related macular degeneration, or retinitis pigmentosa. CD34+ cells were isolated under Good Manufacturing Practice conditions from the mononuclear cellular fraction of the BM aspirate using a CliniMACs magnetic cell sorter. After intravitreal CD34+ cell injection, serial ophthalmic examinations, microperimetry/perimetry, fluorescein angiography, electroretinography (ERG), optical coherence tomography (OCT), and adaptive optics OCT were performed during the 6-month follow-up.
Results: A mean of 3.4 million (range, 1-7 million) CD34+ cells were isolated and injected per eye. The therapy was well tolerated with no intraocular inflammation or hyperproliferation. Best-corrected visual acuity and full-field ERG showed no worsening after 6 months. Clinical examination also showed no worsening during follow-up except among age-related macular degeneration subjects in whom mild progression of geographic atrophy was noted in both the study eye and contralateral eye at 6-month follow-up, concurrent with some possible decline on multifocal ERG and microperimetry. Cellular in vivo imaging using adaptive optics OCT showed changes suggestive of new cellular incorporation into the macula of the hereditary macular degeneration study eye.
Conclusions: Intravitreal autologous BM CD34+ cell therapy appears feasible and well tolerated in eyes with ischemic or degenerative retinal conditions and merits further exploration. (ClinicalTrials.gov number, NCT01736059.).
Keywords: OCT; retinal degeneration; retinal imaging; retinal vein occlusion; stem cells.
Copyright 2014 The Association for Research in Vision and Ophthalmology, Inc.
Figures

Similar articles
-
Phase I Study of Intravitreal Injection of Autologous CD34+ Stem Cells from Bone Marrow in Eyes with Vision Loss from Retinitis Pigmentosa.Ophthalmol Sci. 2024 Jul 31;5(1):100589. doi: 10.1016/j.xops.2024.100589. eCollection 2025 Jan-Feb. Ophthalmol Sci. 2024. PMID: 39328826 Free PMC article.
-
Intravitreal Administration of Human Bone Marrow CD34+ Stem Cells in a Murine Model of Retinal Degeneration.Invest Ophthalmol Vis Sci. 2016 Aug 1;57(10):4125-35. doi: 10.1167/iovs.16-19252. Invest Ophthalmol Vis Sci. 2016. PMID: 27537262 Free PMC article.
-
Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial.Retina. 2011 Jun;31(6):1207-14. doi: 10.1097/IAE.0b013e3181f9c242. Retina. 2011. PMID: 21293313 Clinical Trial.
-
Advances in bone marrow stem cell therapy for retinal dysfunction.Prog Retin Eye Res. 2017 Jan;56:148-165. doi: 10.1016/j.preteyeres.2016.10.002. Epub 2016 Oct 23. Prog Retin Eye Res. 2017. PMID: 27784628 Free PMC article. Review.
-
Stem cell therapy for retinal disease.Curr Opin Ophthalmol. 2012 May;23(3):226-34. doi: 10.1097/ICU.0b013e328352407d. Curr Opin Ophthalmol. 2012. PMID: 22450217 Review.
Cited by
-
Mesenchymal Stem Cell-Based Therapy for Retinal Degenerative Diseases: Experimental Models and Clinical Trials.Cells. 2021 Mar 7;10(3):588. doi: 10.3390/cells10030588. Cells. 2021. PMID: 33799995 Free PMC article. Review.
-
Stem cells: a promising candidate to treat neurological disorders.Neural Regen Res. 2018 Jul;13(7):1294-1304. doi: 10.4103/1673-5374.235085. Neural Regen Res. 2018. PMID: 30028342 Free PMC article. Review.
-
Subretinal versus intravitreal administration of human CD34+ bone marrow-derived stem cells in a rat model of inherited retinal degeneration.Ann Transl Med. 2021 Aug;9(15):1275. doi: 10.21037/atm-20-4662. Ann Transl Med. 2021. PMID: 34532412 Free PMC article.
-
Endothelial and Astrocytic Support by Human Bone Marrow Stem Cell Grafts into Symptomatic ALS Mice towards Blood-Spinal Cord Barrier Repair.Sci Rep. 2017 Apr 13;7(1):884. doi: 10.1038/s41598-017-00993-0. Sci Rep. 2017. PMID: 28408761 Free PMC article.
-
Therapeutic effect against retinal neovascularization in a mouse model of oxygen-induced retinopathy: bone marrow-derived mesenchymal stem cells versus Conbercept.BMC Ophthalmol. 2020 Jan 6;20(1):7. doi: 10.1186/s12886-019-1292-x. BMC Ophthalmol. 2020. PMID: 31906900 Free PMC article.
References
-
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004; 291: 1900–1901. - PubMed
-
- Yanuzzi LA. The Retinal Atlas. United Kingdom: Elsevier Saunders; 2010: 63–68.
-
- Mitchell P, Smithe W, Chang A. Prevalence and association of retinal vein occlusion in Australia: the Blue Mountain Eye Study. Arch Ophthalmol. 1996; 114: 1243–1247. - PubMed
-
- Lu B, Malcuit C, Wang S, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009; 27: 2126–2135. - PubMed
-
- Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009; 458: 126–131. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

