Glucokinase activity in the arcuate nucleus regulates glucose intake
- PMID: 25485685
- PMCID: PMC4382228
- DOI: 10.1172/JCI77172
Glucokinase activity in the arcuate nucleus regulates glucose intake
Abstract
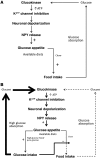
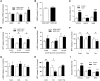
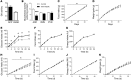
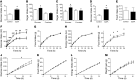
The brain relies on a constant supply of glucose, its primary fuel, for optimal function. A taste-independent mechanism within the CNS that promotes glucose delivery to the brain has been postulated to maintain glucose homeostasis; however, evidence for such a mechanism is lacking. Here, we determined that glucokinase activity within the hypothalamic arcuate nucleus is involved in regulation of dietary glucose intake. In fasted rats, glucokinase activity was specifically increased in the arcuate nucleus but not other regions of the hypothalamus. Moreover, pharmacologic and genetic activation of glucokinase in the arcuate nucleus of rodent models increased glucose ingestion, while decreased arcuate nucleus glucokinase activity reduced glucose intake. Pharmacologic targeting of potential downstream glucokinase effectors revealed that ATP-sensitive potassium channel and P/Q calcium channel activity are required for glucokinase-mediated glucose intake. Additionally, altered glucokinase activity affected release of the orexigenic neurotransmitter neuropeptide Y in response to glucose. Together, our results suggest that glucokinase activity in the arcuate nucleus specifically regulates glucose intake and that appetite for glucose is an important driver of overall food intake. Arcuate nucleus glucokinase activation may represent a CNS mechanism that underlies the oft-described phenomena of the "sweet tooth" and carbohydrate craving.
Figures

Similar articles
-
Hypothalamic arcuate nucleus glucokinase regulates insulin secretion and glucose homeostasis.Diabetes Obes Metab. 2018 Sep;20(9):2246-2254. doi: 10.1111/dom.13359. Epub 2018 Jun 12. Diabetes Obes Metab. 2018. PMID: 29748994 Free PMC article.
-
Arcuate nucleus glucokinase and dietary glucose intake.Oncotarget. 2015 Aug 21;6(24):19926-7. doi: 10.18632/oncotarget.5138. Oncotarget. 2015. PMID: 26343370 Free PMC article. No abstract available.
-
Pharmacological characterization and appetite suppressive properties of BMS-193885, a novel and selective neuropeptide Y(1) receptor antagonist.Eur J Pharmacol. 2008 Aug 20;590(1-3):224-32. doi: 10.1016/j.ejphar.2008.06.032. Epub 2008 Jun 12. Eur J Pharmacol. 2008. PMID: 18573246
-
Glucosensing neurons do more than just sense glucose.Int J Obes Relat Metab Disord. 2001 Dec;25 Suppl 5:S68-72. doi: 10.1038/sj.ijo.0801916. Int J Obes Relat Metab Disord. 2001. PMID: 11840219 Review.
-
Metabolic sensors: viewing glucosensing neurons from a broader perspective.Physiol Behav. 2002 Jul;76(3):397-401. doi: 10.1016/s0031-9384(02)00763-1. Physiol Behav. 2002. PMID: 12117576 Review.
Cited by
-
Longitudinal changes in EEG power, sleep cycles and behaviour in a tau model of neurodegeneration.Alzheimers Res Ther. 2020 Jul 15;12(1):84. doi: 10.1186/s13195-020-00651-0. Alzheimers Res Ther. 2020. PMID: 32669112 Free PMC article.
-
Exenatide Regulates Cerebral Glucose Metabolism in Brain Areas Associated With Glucose Homeostasis and Reward System.Diabetes. 2015 Oct;64(10):3406-12. doi: 10.2337/db14-1718. Epub 2015 Jun 26. Diabetes. 2015. PMID: 26116695 Free PMC article. Clinical Trial.
-
Hypothalamic arcuate nucleus glucokinase regulates insulin secretion and glucose homeostasis.Diabetes Obes Metab. 2018 Sep;20(9):2246-2254. doi: 10.1111/dom.13359. Epub 2018 Jun 12. Diabetes Obes Metab. 2018. PMID: 29748994 Free PMC article.
-
Continuous monitoring devices and seizure patterns by glucose, time and lateralized seizure onset.Epilepsy Behav Case Rep. 2018 Mar 21;10:65-70. doi: 10.1016/j.ebcr.2018.03.001. eCollection 2018. Epilepsy Behav Case Rep. 2018. PMID: 30073145 Free PMC article.
-
Distribution of corticotropin-releasing factor receptor 1 in the developing mouse forebrain: A novel sex difference revealed in the rostral periventricular hypothalamus.Neuroscience. 2017 Oct 11;361:167-178. doi: 10.1016/j.neuroscience.2017.08.016. Epub 2017 Aug 18. Neuroscience. 2017. PMID: 28823817 Free PMC article.
References
-
- Jacobs HL. Some physical, metabolic, and sensory components in the appetite for glucose. Am J Physiol. 1962;203:1043–1054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/E52708X/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 090792/Z/09/A/WT_/Wellcome Trust/United Kingdom
- BB/I00842X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_U120027537/MRC_/Medical Research Council/United Kingdom
- BB/I00842X/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

