Uncoupling protein-2 attenuates palmitoleate protection against the cytotoxic production of mitochondrial reactive oxygen species in INS-1E insulinoma cells
- PMID: 25482405
- PMCID: PMC4309862
- DOI: 10.1016/j.redox.2014.11.009
Uncoupling protein-2 attenuates palmitoleate protection against the cytotoxic production of mitochondrial reactive oxygen species in INS-1E insulinoma cells
Abstract
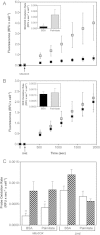
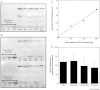
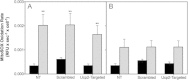
High glucose and fatty acid levels impair pancreatic beta cell function. We have recently shown that palmitate-induced loss of INS-1E insulinoma cells is related to increased reactive oxygen species (ROS) production as both toxic effects are prevented by palmitoleate. Here we show that palmitate-induced ROS are mostly mitochondrial: oxidation of MitoSOX, a mitochondria-targeted superoxide probe, is increased by palmitate, whilst oxidation of the equivalent non-targeted probe is unaffected. Moreover, mitochondrial respiratory inhibition with antimycin A stimulates palmitate-induced MitoSOX oxidation. We also show that palmitate does not change the level of mitochondrial uncoupling protein-2 (UCP2) and that UCP2 knockdown does not affect palmitate-induced MitoSOX oxidation. Palmitoleate does not influence MitoSOX oxidation in INS-1E cells ±UCP2 and largely prevents the palmitate-induced effects. Importantly, UCP2 knockdown amplifies the preventive effect of palmitoleate on palmitate-induced ROS. Consistently, viability effects of palmitate and palmitoleate are similar between cells ±UCP2, but UCP2 knockdown significantly augments the palmitoleate protection against palmitate-induced cell loss at high glucose. We conclude that UCP2 neither mediates palmitate-induced mitochondrial ROS generation and the associated cell loss, nor protects against these deleterious effects. Instead, UCP2 dampens palmitoleate protection against palmitate toxicity.
Keywords: Cytoprotection; Glucolipotoxicity; INS-1E insulinoma cells; Mitochondrial dysfunction; Non-esterified fatty acids; Obesity; Pancreatic beta cells; Reactive oxygen species; Type 2 diabetes; Uncoupling protein-2 (UCP2).
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Measuring mitochondrial uncoupling protein-2 level and activity in insulinoma cells.Methods Enzymol. 2013;528:257-67. doi: 10.1016/B978-0-12-405881-1.00015-X. Methods Enzymol. 2013. PMID: 23849870
-
Uncoupling protein-2 mediates the protective action of berberine against oxidative stress in rat insulinoma INS-1E cells and in diabetic mouse islets.Br J Pharmacol. 2014 Jul;171(13):3246-54. doi: 10.1111/bph.12666. Br J Pharmacol. 2014. PMID: 24588674 Free PMC article.
-
Novel insights into pancreatic β-cell glucolipotoxicity from real-time functional analysis of mitochondrial energy metabolism in INS-1E insulinoma cells.Biochem J. 2013 Dec 15;456(3):417-26. doi: 10.1042/BJ20131002. Biochem J. 2013. PMID: 24099598
-
Mitochondrial uncoupling protein 2 in pancreatic β-cells.Diabetes Obes Metab. 2010 Oct;12 Suppl 2:134-40. doi: 10.1111/j.1463-1326.2010.01264.x. Diabetes Obes Metab. 2010. PMID: 21029310 Review.
-
Antioxidant and regulatory role of mitochondrial uncoupling protein UCP2 in pancreatic beta-cells.Physiol Res. 2014;63(Suppl 1):S73-91. doi: 10.33549/physiolres.932633. Physiol Res. 2014. PMID: 24564667 Review.
Cited by
-
Redox Status as a Key Driver of Healthy Pancreatic Beta-Cells.Physiol Res. 2024 Aug 30;73(S1):S139-S152. doi: 10.33549/physiolres.935259. Epub 2024 Apr 22. Physiol Res. 2024. PMID: 38647167 Free PMC article. Review.
-
Protocol for a randomized placebo-controlled clinical trial using pure palmitoleic acid to ameliorate insulin resistance and lipogenesis in overweight and obese subjects with prediabetes.Front Endocrinol (Lausanne). 2024 Jan 19;14:1306528. doi: 10.3389/fendo.2023.1306528. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38313838 Free PMC article.
-
Pro-inflammatory cytokines attenuate glucose-stimulated insulin secretion from INS-1E insulinoma cells by restricting mitochondrial pyruvate oxidation capacity - Novel mechanistic insight from real-time analysis of oxidative phosphorylation.PLoS One. 2018 Jun 28;13(6):e0199505. doi: 10.1371/journal.pone.0199505. eCollection 2018. PLoS One. 2018. PMID: 29953508 Free PMC article.
-
Mitochondrial uncoupling protein-2 is not involved in palmitate-induced impairment of glucose-stimulated insulin secretion in INS-1E insulinoma cells and is not needed for the amplification of insulin release.Biochem Biophys Rep. 2015 May;1:8-15. doi: 10.1016/j.bbrep.2015.03.008. Biochem Biophys Rep. 2015. PMID: 26339685 Free PMC article.
-
Beta-aminoisobutyric acid is released by contracting human skeletal muscle and lowers insulin release from INS-1 832/3 cells by mediating mitochondrial energy metabolism.Metabol Open. 2020 Aug 22;7:100053. doi: 10.1016/j.metop.2020.100053. eCollection 2020 Sep. Metabol Open. 2020. PMID: 32924003 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

