Pluripotent stem cell energy metabolism: an update
- PMID: 25476451
- PMCID: PMC4337063
- DOI: 10.15252/embj.201490446
Pluripotent stem cell energy metabolism: an update
Abstract
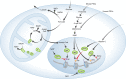
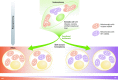
Recent studies link changes in energy metabolism with the fate of pluripotent stem cells (PSCs). Safe use of PSC derivatives in regenerative medicine requires an enhanced understanding and control of factors that optimize in vitro reprogramming and differentiation protocols. Relative shifts in metabolism from naïve through "primed" pluripotent states to lineage-directed differentiation place variable demands on mitochondrial biogenesis and function for cell types with distinct energetic and biosynthetic requirements. In this context, mitochondrial respiration, network dynamics, TCA cycle function, and turnover all have the potential to influence reprogramming and differentiation outcomes. Shifts in cellular metabolism affect enzymes that control epigenetic configuration, which impacts chromatin reorganization and gene expression changes during reprogramming and differentiation. Induced PSCs (iPSCs) may have utility for modeling metabolic diseases caused by mutations in mitochondrial DNA, for which few disease models exist. Here, we explore key features of PSC energy metabolism research in mice and man and the impact this work is starting to have on our understanding of early development, disease modeling, and potential therapeutic applications.
Keywords: differentiation; epigenetics; metabolism; mitochondria; pluripotency.
© 2014 The Authors.
Figures

Similar articles
-
Mechanisms of the Metabolic Shift during Somatic Cell Reprogramming.Int J Mol Sci. 2019 May 7;20(9):2254. doi: 10.3390/ijms20092254. Int J Mol Sci. 2019. PMID: 31067778 Free PMC article. Review.
-
Connecting Mitochondria, Metabolism, and Stem Cell Fate.Stem Cells Dev. 2015 Sep 1;24(17):1957-71. doi: 10.1089/scd.2015.0117. Epub 2015 Jul 2. Stem Cells Dev. 2015. PMID: 26134242 Free PMC article. Review.
-
Mitochondrial function in pluripotent stem cells and cellular reprogramming.Gerontology. 2014;60(2):174-82. doi: 10.1159/000355050. Epub 2013 Nov 19. Gerontology. 2014. PMID: 24281332 Review.
-
Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal.Cell Stem Cell. 2012 Nov 2;11(5):589-95. doi: 10.1016/j.stem.2012.10.005. Cell Stem Cell. 2012. PMID: 23122286 Free PMC article.
-
Interference with the mitochondrial bioenergetics fuels reprogramming to pluripotency via facilitation of the glycolytic transition.Int J Biochem Cell Biol. 2013 Nov;45(11):2512-8. doi: 10.1016/j.biocel.2013.07.023. Epub 2013 Aug 9. Int J Biochem Cell Biol. 2013. PMID: 23939289
Cited by
-
MiR200 and miR302: Two Big Families Influencing Stem Cell Behavior.Molecules. 2018 Jan 30;23(2):282. doi: 10.3390/molecules23020282. Molecules. 2018. PMID: 29385685 Free PMC article. Review.
-
The Force Is Strong with This One: Metabolism (Over)powers Stem Cell Fate.Trends Cell Biol. 2018 Jul;28(7):551-559. doi: 10.1016/j.tcb.2018.02.007. Epub 2018 Mar 16. Trends Cell Biol. 2018. PMID: 29555207 Free PMC article. Review.
-
Redox Homeostasis and Regulation in Pluripotent Stem Cells: Uniqueness or Versatility?Int J Mol Sci. 2021 Oct 11;22(20):10946. doi: 10.3390/ijms222010946. Int J Mol Sci. 2021. PMID: 34681606 Free PMC article. Review.
-
Metabolic control of DNA methylation in naive pluripotent cells.Nat Genet. 2021 Feb;53(2):215-229. doi: 10.1038/s41588-020-00770-2. Epub 2021 Feb 1. Nat Genet. 2021. PMID: 33526924 Free PMC article.
-
Modifying the Mitochondrial Genome.Cell Metab. 2016 May 10;23(5):785-96. doi: 10.1016/j.cmet.2016.04.004. Cell Metab. 2016. PMID: 27166943 Free PMC article. Review.
References
-
- Adamo A, Sese B, Boue S, Castano J, Paramonov I, Barrero MJ, Belmonte JCI. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol. 2011;13:652–659. - PubMed
-
- Alavi MV, Bette S, Schimpf S, Schuettauf F, Schraermeyer U, Wehrl HF, Ruttiger L, Beck SC, Tonagel F, Pichler BJ, Knipper M, Peters T, Laufs J, Wissinger B. A splice site mutation in the murine Opa1 gene features pathology of autosomal dominant optic atrophy. Brain. 2007;130:1029–1042. - PubMed
-
- Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R, Przyborski S, Lako M. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells. 2010;28:661–673. - PubMed
Publication types
MeSH terms
Grants and funding
- CA156674/CA/NCI NIH HHS/United States
- CA90571/CA/NCI NIH HHS/United States
- GM114188/GM/NIGMS NIH HHS/United States
- R01 CA185189/CA/NCI NIH HHS/United States
- R01 CA090571/CA/NCI NIH HHS/United States
- CA185189/CA/NCI NIH HHS/United States
- R01 GM073981/GM/NIGMS NIH HHS/United States
- GM073981/GM/NIGMS NIH HHS/United States
- R01 CA156674/CA/NCI NIH HHS/United States
- T32 GM007185/GM/NIGMS NIH HHS/United States
- R01 GM114188/GM/NIGMS NIH HHS/United States
- P01 GM081621/GM/NIGMS NIH HHS/United States
- P01GM081621/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

