Tetraspanin (TSP-17) protects dopaminergic neurons against 6-OHDA-induced neurodegeneration in C. elegans
- PMID: 25474638
- PMCID: PMC4256090
- DOI: 10.1371/journal.pgen.1004767
Tetraspanin (TSP-17) protects dopaminergic neurons against 6-OHDA-induced neurodegeneration in C. elegans
Abstract
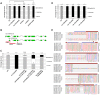
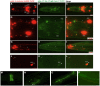
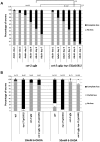
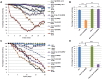
Parkinson's disease (PD), the second most prevalent neurodegenerative disease after Alzheimer's disease, is linked to the gradual loss of dopaminergic neurons in the substantia nigra. Disease loci causing hereditary forms of PD are known, but most cases are attributable to a combination of genetic and environmental risk factors. Increased incidence of PD is associated with rural living and pesticide exposure, and dopaminergic neurodegeneration can be triggered by neurotoxins such as 6-hydroxydopamine (6-OHDA). In C. elegans, this drug is taken up by the presynaptic dopamine reuptake transporter (DAT-1) and causes selective death of the eight dopaminergic neurons of the adult hermaphrodite. Using a forward genetic approach to find genes that protect against 6-OHDA-mediated neurodegeneration, we identified tsp-17, which encodes a member of the tetraspanin family of membrane proteins. We show that TSP-17 is expressed in dopaminergic neurons and provide genetic, pharmacological and biochemical evidence that it inhibits DAT-1, thus leading to increased 6-OHDA uptake in tsp-17 loss-of-function mutants. TSP-17 also protects against toxicity conferred by excessive intracellular dopamine. We provide genetic and biochemical evidence that TSP-17 acts partly via the DOP-2 dopamine receptor to negatively regulate DAT-1. tsp-17 mutants also have subtle behavioral phenotypes, some of which are conferred by aberrant dopamine signaling. Incubating mutant worms in liquid medium leads to swimming-induced paralysis. In the L1 larval stage, this phenotype is linked to lethality and cannot be rescued by a dop-3 null mutant. In contrast, mild paralysis occurring in the L4 larval stage is suppressed by dop-3, suggesting defects in dopaminergic signaling. In summary, we show that TSP-17 protects against neurodegeneration and has a role in modulating behaviors linked to dopamine signaling.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Mutations in Caenorhabditis elegans neuroligin-like glit-1, the apoptosis pathway and the calcium chaperone crt-1 increase dopaminergic neurodegeneration after 6-OHDA treatment.PLoS Genet. 2018 Jan 18;14(1):e1007106. doi: 10.1371/journal.pgen.1007106. eCollection 2018 Jan. PLoS Genet. 2018. PMID: 29346364 Free PMC article.
-
Alpha-linolenic acid suppresses dopaminergic neurodegeneration induced by 6-OHDA in C. elegans.Physiol Behav. 2015 Nov 1;151:563-9. doi: 10.1016/j.physbeh.2015.08.025. Epub 2015 Aug 20. Physiol Behav. 2015. PMID: 26300470
-
6-OHDA-induced dopaminergic neurodegeneration in Caenorhabditis elegans is promoted by the engulfment pathway and inhibited by the transthyretin-related protein TTR-33.PLoS Genet. 2018 Jan 18;14(1):e1007125. doi: 10.1371/journal.pgen.1007125. eCollection 2018 Jan. PLoS Genet. 2018. PMID: 29346382 Free PMC article.
-
The Caenorhabditis elegans dopaminergic system: opportunities for insights into dopamine transport and neurodegeneration.Annu Rev Pharmacol Toxicol. 2003;43:521-44. doi: 10.1146/annurev.pharmtox.43.100901.135934. Epub 2002 Jan 10. Annu Rev Pharmacol Toxicol. 2003. PMID: 12415122 Review.
-
Modeling Parkinson's Disease in C. elegans.J Parkinsons Dis. 2018;8(1):17-32. doi: 10.3233/JPD-171258. J Parkinsons Dis. 2018. PMID: 29480229 Free PMC article. Review.
Cited by
-
6-hydroxydopamine (6-OHDA) Oxidative Stress Assay for Observing Dopaminergic Neuron Loss in Caenorhabditis elegans.Bio Protoc. 2018 Sep 20;8(18):e3025. doi: 10.21769/BioProtoc.3025. Bio Protoc. 2018. PMID: 30406156 Free PMC article.
-
Mutations in Caenorhabditis elegans neuroligin-like glit-1, the apoptosis pathway and the calcium chaperone crt-1 increase dopaminergic neurodegeneration after 6-OHDA treatment.PLoS Genet. 2018 Jan 18;14(1):e1007106. doi: 10.1371/journal.pgen.1007106. eCollection 2018 Jan. PLoS Genet. 2018. PMID: 29346364 Free PMC article.
-
Chronic high-sugar diet in adulthood protects Caenorhabditis elegans from 6-OHDA-induced dopaminergic neurodegeneration.BMC Biol. 2023 Nov 10;21(1):252. doi: 10.1186/s12915-023-01733-9. BMC Biol. 2023. PMID: 37950228 Free PMC article.
-
Acoustic streaming enabled moderate swimming exercise reduces neurodegeneration in C. elegans.Sci Adv. 2023 Feb 22;9(8):eadf5056. doi: 10.1126/sciadv.adf5056. Epub 2023 Feb 22. Sci Adv. 2023. PMID: 36812319 Free PMC article.
-
Genetic Silencing of Fatty Acid Desaturases Modulates α-Synuclein Toxicity and Neuronal Loss in Parkinson-Like Models of C. elegans.Front Aging Neurosci. 2019 Aug 6;11:207. doi: 10.3389/fnagi.2019.00207. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31447665 Free PMC article.
References
-
- Calne DB, Langston JW (1983) Aetiology of Parkinson's disease. Lancet 2: 1457–1459. - PubMed
-
- Zigmond MJ, Burke RE (2002) pathophysiology of parkinson's disease. Fifth generation of progess American college of Neuropsychopharmacology Williams and Wilkens 1781–1794.
-
- Nass R, Blakely RD (2003) The Caenorhabditis elegans dopaminergic system: opportunities for insights into dopamine transport and neurodegeneration. Annual review of pharmacology and toxicology 43: 521–544. - PubMed
-
- Chase TN (1997) A gene for Parkinson disease. Archives of neurology 54: 1156–1157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

