The Usefulness of a Novel Screening Kit for Colorectal Cancer Using the Immunochromatographic Fecal Tumor M2 Pyruvate Kinase Test
- PMID: 25473070
- PMCID: PMC4562782
- DOI: 10.5009/gnl13457
The Usefulness of a Novel Screening Kit for Colorectal Cancer Using the Immunochromatographic Fecal Tumor M2 Pyruvate Kinase Test
Abstract
Background/aims: M2 pyruvate kinase (M2-PK) is an enzyme that is produced in undifferentiated and proliferating tissues. This study aims to evaluate the usefulness of the immunochromatographic M2 pyruvate kinase (iM2-PK) for the screening of colorectal cancer (CRC) and premalignant lesions.
Methods: Healthy volunteers and patients with colorectal neoplasia were enrolled in six academic hospitals in the capital province of Korea. The iM2-PK value was compared with the immunochromatographic fecal occult blood test (iFOBT) and fecal tumor M2-PK enzyme-linked immunosorbent assay (ELISA).
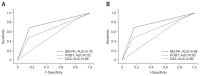
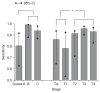
Results: A total of 323 subjects were enrolled. The sensitivity of iM2-PK for CRC was 92.8%, which was superior to iFOBT (47.5%, p<0.0001). For adenomatous lesions, the sensitivity of iM2-PK was 69.4%, which was also superior to iFOBT (12.1%, p<0.001). Compared with M2-PK ELISA, iM2-PK exhibited significantly enhanced sensitivity for CRC (97.5% vs 80.0%, p=0.0289). The sensitivity of iM2-PK was higher in advanced stages of CRC compared with cancers confined to the mucosa and submucosa (p<0.05). However, lymph node metastasis had no influence on the sensitivity of iM2-PK.
Conclusions: The iM2-PK exhibited increased sensitivity for identifying CRC and adenomatous lesions compared with iFOBT. Given its rapid results and convenience, CRC screening using iM2-PK is promising.
Keywords: Colorectal neoplasms; Fecal occult blood test; Mass screening; Tumor M2 pyruvate kinase.
Figures
Similar articles
-
Colorectal cancer detection in an asymptomatic population: fecal immunochemical test for hemoglobin vs. fecal M2-type pyruvate kinase.Biochem Med (Zagreb). 2016;26(1):114-20. doi: 10.11613/BM.2016.012. Biochem Med (Zagreb). 2016. PMID: 26981025 Free PMC article.
-
Comparison of an established simple office-based immunological FOBT with fecal tumor pyruvate kinase type M2 (M2-PK) for colorectal cancer screening: prospective multicenter study.Am J Gastroenterol. 2008 Jun;103(6):1496-504. doi: 10.1111/j.1572-0241.2008.01824.x. Epub 2008 May 28. Am J Gastroenterol. 2008. PMID: 18510609 Clinical Trial.
-
Tumor pyruvate kinase isoenzyme type M2 and immunochemical fecal occult blood test: performance in screening for colorectal cancer.Eur J Gastroenterol Hepatol. 2007 Oct;19(10):878-82. doi: 10.1097/MEG.0b013e3282cfa49c. Eur J Gastroenterol Hepatol. 2007. PMID: 17873612
-
[Pyruvate kinase M2 (tumor M2-PK) as a screening tool for colorectal cancer (CRC). A review of current published data].Z Gastroenterol. 2005 Dec;43(12):1313-7. doi: 10.1055/s-2005-858657. Z Gastroenterol. 2005. PMID: 16315127 Review. German.
-
Stool test for colorectal cancer screening--it's time to move!Clin Lab. 2008;54(11-12):473-84. Clin Lab. 2008. PMID: 19216253 Review.
Cited by
-
Identifying Novel Biomarkers Ready for Evaluation in Low-Prevalence Populations for the Early Detection of Lower Gastrointestinal Cancers: A Systematic Review and Meta-Analysis.Adv Ther. 2021 Jun;38(6):3032-3065. doi: 10.1007/s12325-021-01645-6. Epub 2021 Apr 27. Adv Ther. 2021. PMID: 33907946 Free PMC article.
-
Comparison of faecal protein biomarkers' diagnostic accuracy for colorectal advanced neoplasms: a systematic review and meta-analysis.Sci Rep. 2022 Feb 16;12(1):2623. doi: 10.1038/s41598-022-06689-4. Sci Rep. 2022. PMID: 35173276 Free PMC article.
-
The Importance of Evaluating Serum Levels of Tumor Markers M2-PK and Inhibin A in Patients Undergoing Colonoscopy.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231194492. doi: 10.1177/15330338231194492. Technol Cancer Res Treat. 2023. PMID: 37574835 Free PMC article.
-
Diagnostic Accuracy of Five Different Fecal Markers for the Detection of Precancerous and Cancerous Lesions of the Colorectum.Mediators Inflamm. 2016;2016:2492081. doi: 10.1155/2016/2492081. Epub 2016 Jun 16. Mediators Inflamm. 2016. PMID: 27413251 Free PMC article.
-
Colorectal cancer detection in an asymptomatic population: fecal immunochemical test for hemoglobin vs. fecal M2-type pyruvate kinase.Biochem Med (Zagreb). 2016;26(1):114-20. doi: 10.11613/BM.2016.012. Biochem Med (Zagreb). 2016. PMID: 26981025 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

