Oral administration of a fusion protein between the cholera toxin B subunit and the 42-amino acid isoform of amyloid-β peptide produced in silkworm pupae protects against Alzheimer's disease in mice
- PMID: 25469702
- PMCID: PMC4254457
- DOI: 10.1371/journal.pone.0113585
Oral administration of a fusion protein between the cholera toxin B subunit and the 42-amino acid isoform of amyloid-β peptide produced in silkworm pupae protects against Alzheimer's disease in mice
Abstract
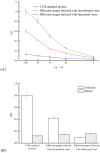
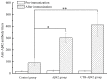
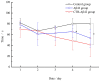
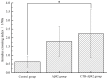
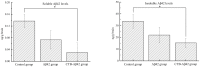
A key molecule in the pathogenesis of Alzheimer's disease (AD) is a 42-amino acid isoform of the amyloid-β peptide (Aβ42), which is the most toxic element of senile plaques. In this study, to develop an edible, safe, low-cost vaccine for AD, a cholera toxin B subunit (CTB)-Aβ42 fusion protein was successfully expressed in silkworm pupae. We tested the silkworm pupae-derived oral vaccination containing CTB-Aβ42 in a transgenic mouse model of AD. Anti-Aβ42 antibodies were induced in these mice, leading to a decreased Aβ deposition in the brain. We also found that the oral administration of the silk worm pupae vaccine improved the memory and cognition of mice, as assessed using a water maze test. These results suggest that the new edible CTB-Aβ42 silkworm pupae-derived vaccine has potential clinical application in the prevention of AD.
Conflict of interest statement
Figures

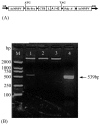
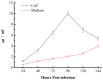
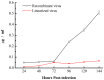
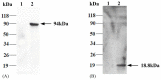

Similar articles
-
Effects of a 15-amino-acid isoform of amyloid- β expressed by silkworm pupae on B6C3-Tg Alzheimer's disease transgenic mice.J Biotechnol. 2019 Apr 20;296:83-92. doi: 10.1016/j.jbiotec.2019.03.013. Epub 2019 Mar 18. J Biotechnol. 2019. PMID: 30898688
-
A novel recombinant adeno-associated virus vaccine reduces behavioral impairment and beta-amyloid plaques in a mouse model of Alzheimer's disease.Neurobiol Dis. 2003 Dec;14(3):365-79. doi: 10.1016/j.nbd.2003.07.005. Neurobiol Dis. 2003. PMID: 14678754
-
Effect of rice-expressed amyloid β in the Tg2576 Alzheimer's disease transgenic mouse model.Vaccine. 2011 Aug 26;29(37):6252-8. doi: 10.1016/j.vaccine.2011.06.073. Epub 2011 Jul 2. Vaccine. 2011. PMID: 21726591
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
[Vaccination therapy for Alzheimer's disease].Rinsho Shinkeigaku. 2009 Nov;49(11):848-50. doi: 10.5692/clinicalneurol.49.848. Rinsho Shinkeigaku. 2009. PMID: 20030228 Review. Japanese.
Cited by
-
Silkworm Pupae: A Functional Food with Health Benefits for Humans.Foods. 2022 May 28;11(11):1594. doi: 10.3390/foods11111594. Foods. 2022. PMID: 35681343 Free PMC article. Review.
-
Nutritional aspects and dietary benefits of "Silkworms": Current scenario and future outlook.Front Nutr. 2023 Jan 19;10:1121508. doi: 10.3389/fnut.2023.1121508. eCollection 2023. Front Nutr. 2023. PMID: 36742434 Free PMC article. Review.
-
Cholera Toxin Subunit B as Adjuvant--An Accelerator in Protective Immunity and a Break in Autoimmunity.Vaccines (Basel). 2015 Jul 24;3(3):579-96. doi: 10.3390/vaccines3030579. Vaccines (Basel). 2015. PMID: 26350596 Free PMC article. Review.
References
-
- Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81:741–766. - PubMed
-
- Schenk D, Games D, Seubert P (2001) Potential treatment opportunities for Alzheimer's disease through inhibition of secretases and Abeta immunization. J Mol Neurosci 17:259–267. - PubMed
-
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, et al. (2002) Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci 5:452–457. - PubMed
-
- Arendash GW, Gordon MN, Diamond DM, Austin LA, Hatcher JM, et al. (2001) Behavioral assessment of Alzheimer's transgenic mice following long-term Abeta vaccination: task specificity and correlations between Abeta deposition and spatial memory. DNA Cell Biol 20:737–744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

