Control of pathogenic effector T-cell activities in situ by PD-L1 expression on respiratory inflammatory dendritic cells during respiratory syncytial virus infection
- PMID: 25465101
- PMCID: PMC4632244
- DOI: 10.1038/mi.2014.106
Control of pathogenic effector T-cell activities in situ by PD-L1 expression on respiratory inflammatory dendritic cells during respiratory syncytial virus infection
Abstract
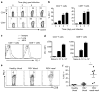
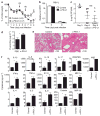
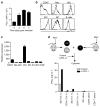
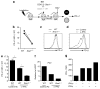
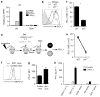
Respiratory syncytial virus (RSV) infection is a leading cause of severe lower respiratory tract illness in young infants, the elderly and immunocompromised individuals. We demonstrate here that the co-inhibitory molecule programmed cell death 1 (PD-1) is selectively upregulated on T cells within the respiratory tract during both murine and human RSV infection. Importantly, the interaction of PD-1 with its ligand PD-L1 is vital to restrict the pro-inflammatory activities of lung effector T cells in situ, thereby inhibiting the development of excessive pulmonary inflammation and injury during RSV infection. We further identify that PD-L1 expression on lung inflammatory dendritic cells is critical to suppress inflammatory T-cell activities, and an interferon-STAT1-IRF1 axis is responsible for increased PD-L1 expression on lung inflammatory dendritic cells. Our findings suggest a potentially critical role of PD-L1 and PD-1 interactions in the lung for controlling host inflammatory responses and disease progression in clinical RSV infection.
Conflict of interest statement
The authors declared not conflict of interest.
Figures
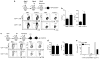
Similar articles
-
Differential response of BDCA-1+ and BDCA-3+ myeloid dendritic cells to respiratory syncytial virus infection.Respir Res. 2013 Jul 5;14(1):71. doi: 10.1186/1465-9921-14-71. Respir Res. 2013. PMID: 23829893 Free PMC article.
-
Sirtuin 1 Regulates Dendritic Cell Activation and Autophagy during Respiratory Syncytial Virus-Induced Immune Responses.J Immunol. 2015 Aug 15;195(4):1637-46. doi: 10.4049/jimmunol.1500326. Epub 2015 Jul 8. J Immunol. 2015. PMID: 26157176 Free PMC article.
-
Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection.J Virol. 2014 Aug;88(16):9350-60. doi: 10.1128/JVI.00818-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920801 Free PMC article.
-
Impairment of T cell immunity by the respiratory syncytial virus: targeting virulence mechanisms for therapy and prophylaxis.Curr Med Chem. 2009;16(34):4609-25. doi: 10.2174/092986709789760724. Curr Med Chem. 2009. PMID: 19903147 Review.
-
The CD4 T cell response to respiratory syncytial virus infection.Immunol Res. 2014 Aug;59(1-3):109-17. doi: 10.1007/s12026-014-8540-1. Immunol Res. 2014. PMID: 24838148 Review.
Cited by
-
Genetic absence of PD-L1 does not restore CD8+ T cell function during respiratory virus infection and delays virus clearance.J Virol. 2024 Oct 22;98(10):e0079724. doi: 10.1128/jvi.00797-24. Epub 2024 Sep 23. J Virol. 2024. PMID: 39311697
-
PD-1 of Sigmodon hispidus: Gene identification, characterization and preliminary evaluation of expression in inactivated RSV vaccine-induced enhanced respiratory disease.Sci Rep. 2019 Aug 12;9(1):11638. doi: 10.1038/s41598-019-48225-x. Sci Rep. 2019. PMID: 31406266 Free PMC article.
-
Effectiveness of Immunotherapy in Non-Small Cell Lung Cancer Patients with a Diagnosis of COPD: Is This a Hidden Prognosticator for Survival and a Risk Factor for Immune-Related Adverse Events?Cancers (Basel). 2024 Mar 22;16(7):1251. doi: 10.3390/cancers16071251. Cancers (Basel). 2024. PMID: 38610929 Free PMC article. Review.
-
Tissue-Resident Macrophages Limit Pulmonary CD8 Resident Memory T Cell Establishment.Front Immunol. 2019 Oct 10;10:2332. doi: 10.3389/fimmu.2019.02332. eCollection 2019. Front Immunol. 2019. PMID: 31681267 Free PMC article.
-
PPAR-γ in Macrophages Limits Pulmonary Inflammation and Promotes Host Recovery following Respiratory Viral Infection.J Virol. 2019 Apr 17;93(9):e00030-19. doi: 10.1128/JVI.00030-19. Print 2019 May 1. J Virol. 2019. PMID: 30787149 Free PMC article.
References
-
- Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006;368:312–322. - PubMed
-
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. - PubMed
-
- Graham BS, Johnson TR, Peebles RS. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology. 2000;48:237–247. - PubMed
-
- Bennett BL, et al. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis. 2007;195:1532–1540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

