Macrophage immunoregulatory pathways in tuberculosis
- PMID: 25453226
- PMCID: PMC4314327
- DOI: 10.1016/j.smim.2014.09.010
Macrophage immunoregulatory pathways in tuberculosis
Abstract
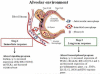
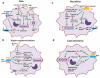
Macrophages, the major host cells harboring Mycobacterium tuberculosis (M.tb), are a heterogeneous cell type depending on their tissue of origin and host they are derived from. Significant discord in macrophage responses to M.tb exists due to differences in M.tb strains and the various types of macrophages used to study tuberculosis (TB). This review will summarize current concepts regarding macrophage responses to M.tb infection, while pointing out relevant differences in experimental outcomes due to the use of divergent model systems. A brief description of the lung environment is included since there is increasing evidence that the alveolar macrophage (AM) has immunoregulatory properties that can delay optimal protective host immune responses. In this context, this review focuses on selected macrophage immunoregulatory pattern recognition receptors (PRRs), cytokines, negative regulators of inflammation, lipid mediators and microRNAs (miRNAs).
Keywords: Innate immunity; Lung; Macrophages; MicroRNAs; Pattern recognition receptors.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Crosstalk between Mycobacterium tuberculosis and the host cell.Semin Immunol. 2014 Dec;26(6):486-96. doi: 10.1016/j.smim.2014.09.002. Epub 2014 Oct 7. Semin Immunol. 2014. PMID: 25303934 Free PMC article. Review.
-
Pro- and anti-inflammatory cytokines in tuberculosis: a two-edged sword in TB pathogenesis.Semin Immunol. 2014 Dec;26(6):543-51. doi: 10.1016/j.smim.2014.09.011. Epub 2014 Nov 8. Semin Immunol. 2014. PMID: 25453229 Review.
-
Deletion of PPARγ in lung macrophages provides an immunoprotective response against M. tuberculosis infection in mice.Tuberculosis (Edinb). 2018 Jul;111:170-177. doi: 10.1016/j.tube.2018.06.012. Epub 2018 Jun 21. Tuberculosis (Edinb). 2018. PMID: 30029904 Free PMC article.
-
Understanding delayed T-cell priming, lung recruitment, and airway luminal T-cell responses in host defense against pulmonary tuberculosis.Clin Dev Immunol. 2012;2012:628293. doi: 10.1155/2012/628293. Epub 2012 Apr 1. Clin Dev Immunol. 2012. PMID: 22545059 Free PMC article. Review.
-
IRAK-M alters the polarity of macrophages to facilitate the survival of Mycobacterium tuberculosis.BMC Microbiol. 2017 Aug 23;17(1):185. doi: 10.1186/s12866-017-1095-2. BMC Microbiol. 2017. PMID: 28835201 Free PMC article.
Cited by
-
Succinate Promotes Phagocytosis of Monocytes/Macrophages in Teleost Fish.Front Mol Biosci. 2021 Apr 15;8:644957. doi: 10.3389/fmolb.2021.644957. eCollection 2021. Front Mol Biosci. 2021. PMID: 33937328 Free PMC article.
-
Association between polymorphisms in mannose-binding lectin 2 gene with pulmonary tuberculosis susceptibility.Hereditas. 2020 Aug 3;157(1):33. doi: 10.1186/s41065-020-00146-w. Hereditas. 2020. PMID: 32746927 Free PMC article.
-
Novel targeting of PEGylated liposomes for codelivery of TGF-β1 siRNA and four antitubercular drugs to human macrophages for the treatment of mycobacterial infection: a quantitative proteomic study.Drug Des Devel Ther. 2015 Aug 7;9:4441-70. doi: 10.2147/DDDT.S79369. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26300629 Free PMC article.
-
Mesenchymal stem cells internalize Mycobacterium tuberculosis through scavenger receptors and restrict bacterial growth through autophagy.Sci Rep. 2017 Nov 8;7(1):15010. doi: 10.1038/s41598-017-15290-z. Sci Rep. 2017. PMID: 29118429 Free PMC article.
-
Itaconate, Arginine, and Gamma-Aminobutyric Acid: A Host Metabolite Triad Protective Against Mycobacterial Infection.Front Immunol. 2022 Feb 4;13:832015. doi: 10.3389/fimmu.2022.832015. eCollection 2022. Front Immunol. 2022. PMID: 35185924 Free PMC article. Review.
References
-
- Bhatt K, Salgame P. Host innate immune response to Mycobacterium tuberculosis. J Clin Immunol. 2007;27:347–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

