Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis
- PMID: 25451236
- PMCID: PMC4384687
- DOI: 10.1016/j.exger.2014.11.018
Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis
Abstract
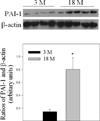
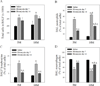
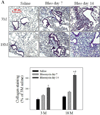
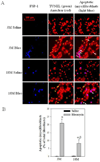
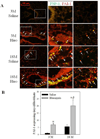
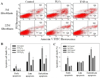
Idiopathic pulmonary fibrosis (IPF) is a fatal lung disorder with unknown cause and no effective treatment. The incidence of and mortality from IPF increase with age, suggesting that advanced age is a major risk factor for IPF. The mechanism underlying the increased susceptibility of the elderly to IPF, however, is unknown. In this study, we show for the first time that the protein level of plasminogen activator inhibitor 1 (PAI-1), a protease inhibitor which plays an essential role in the control of fibrinolysis, was significantly increased with age in mouse lung homogenate and lung fibroblasts. Upon bleomycin challenge, old mice experienced augmented PAI-1 induction and lung fibrosis as compared to young mice. Most interestingly, we show that fewer (myo)fibroblasts underwent apoptosis and more (myo)fibroblasts with increased level of PAI-1 accumulated in the lung of old than in young mice after bleomycin challenge. In vitro studies further demonstrate that fibroblasts isolated from lungs of old mice were resistant to H2O2 and tumor necrosis factor alpha-induced apoptosis and had augmented fibrotic responses to TGF-β1, compared to fibroblasts isolated from young mice. Inhibition of PAI-1 activity with a PAI-1 inhibitor, on the other hand, eliminated the aging-related apoptosis resistance and TGF-β1 sensitivity in isolated fibroblasts. Moreover, we show that knocking down PAI-1 in human lung fibroblasts with PAI-1 siRNA significantly increased their sensitivity to apoptosis and inhibited their responses to TGF-β1. Together, the results suggest that increased PAI-1 expression may underlie the aging-related sensitivity to lung fibrosis in part by protecting fibroblasts from apoptosis.
Keywords: Aging; Fibroblast apoptosis; Idiopathic pulmonary fibrosis; Plasminogen activator inhibitor 1.
Published by Elsevier Inc.
Figures









Similar articles
-
Plasminogen activator inhibitor-1 suppresses profibrotic responses in fibroblasts from fibrotic lungs.J Biol Chem. 2015 Apr 10;290(15):9428-41. doi: 10.1074/jbc.M114.601815. Epub 2015 Feb 3. J Biol Chem. 2015. PMID: 25648892 Free PMC article.
-
Inhibition of Plasminogen Activator Inhibitor-1 Attenuates Transforming Growth Factor-β-Dependent Epithelial Mesenchymal Transition and Differentiation of Fibroblasts to Myofibroblasts.PLoS One. 2016 Feb 9;11(2):e0148969. doi: 10.1371/journal.pone.0148969. eCollection 2016. PLoS One. 2016. PMID: 26859294 Free PMC article.
-
Suppression of plasminogen activator inhibitor-1 by RNA interference attenuates pulmonary fibrosis.Thorax. 2010 Apr;65(4):334-40. doi: 10.1136/thx.2009.119974. Thorax. 2010. PMID: 20388759
-
Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages.Curr Pharm Des. 2007;13(12):1247-56. doi: 10.2174/138161207780618885. Curr Pharm Des. 2007. PMID: 17504233 Review.
-
Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis.Antioxid Redox Signal. 2008 Feb;10(2):303-19. doi: 10.1089/ars.2007.1903. Antioxid Redox Signal. 2008. PMID: 17979497 Free PMC article. Review.
Cited by
-
Simulated in vitro hypoxic conditions from psoriatic arthritis cartilage change plasminogen activating system urokinase and serpine functionality. Reversal of antiapoptotic protection suggests common homeostatic buffering.Postepy Dermatol Alergol. 2022 Oct;39(5):944-952. doi: 10.5114/ada.2022.113405. Epub 2022 Feb 8. Postepy Dermatol Alergol. 2022. PMID: 36457693 Free PMC article.
-
PAI-1 Regulation of TGF-β1-induced Alveolar Type II Cell Senescence, SASP Secretion, and SASP-mediated Activation of Alveolar Macrophages.Am J Respir Cell Mol Biol. 2020 Mar;62(3):319-330. doi: 10.1165/rcmb.2019-0071OC. Am J Respir Cell Mol Biol. 2020. PMID: 31513752 Free PMC article.
-
Cellular Senescence: Pathogenic Mechanisms in Lung Fibrosis.Int J Mol Sci. 2021 Jun 9;22(12):6214. doi: 10.3390/ijms22126214. Int J Mol Sci. 2021. PMID: 34207528 Free PMC article. Review.
-
Systems biology analysis of lung fibrosis-related genes in the bleomycin mouse model.Sci Rep. 2021 Sep 29;11(1):19269. doi: 10.1038/s41598-021-98674-6. Sci Rep. 2021. PMID: 34588506 Free PMC article.
-
COVID-19 and pulmonary fibrosis: A potential role for lung epithelial cells and fibroblasts.Immunol Rev. 2021 Jul;302(1):228-240. doi: 10.1111/imr.12977. Epub 2021 May 24. Immunol Rev. 2021. PMID: 34028807 Free PMC article. Review.
References
-
- Aillaud MF, Pignol F, Alessi MC, Harle JR, Escande M, Mongin M, Juhan-Vague I. Increase in plasma concentration of plasminogen activator inhibitor, fibrinogen, von Willebrand factor, factor VIII:C and in erythrocyte sedimentation rate with age. Thrombosis and haemostasis. 1986;55:330–332. - PubMed
-
- Araki T, Katsura H, Sawabe M, Kida K. A clinical study of idiopathic pulmonary fibrosis based on autopsy studies in elderly patients. Intern Med. 2003;42:483–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

