Mutations in GTPBP3 cause a mitochondrial translation defect associated with hypertrophic cardiomyopathy, lactic acidosis, and encephalopathy
- PMID: 25434004
- PMCID: PMC4259976
- DOI: 10.1016/j.ajhg.2014.10.017
Mutations in GTPBP3 cause a mitochondrial translation defect associated with hypertrophic cardiomyopathy, lactic acidosis, and encephalopathy
Abstract
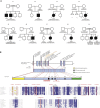
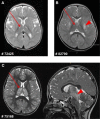
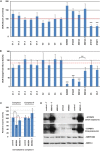
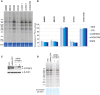
Respiratory chain deficiencies exhibit a wide variety of clinical phenotypes resulting from defective mitochondrial energy production through oxidative phosphorylation. These defects can be caused by either mutations in the mtDNA or mutations in nuclear genes coding for mitochondrial proteins. The underlying pathomechanisms can affect numerous pathways involved in mitochondrial physiology. By whole-exome and candidate gene sequencing, we identified 11 individuals from 9 families carrying compound heterozygous or homozygous mutations in GTPBP3, encoding the mitochondrial GTP-binding protein 3. Affected individuals from eight out of nine families presented with combined respiratory chain complex deficiencies in skeletal muscle. Mutations in GTPBP3 are associated with a severe mitochondrial translation defect, consistent with the predicted function of the protein in catalyzing the formation of 5-taurinomethyluridine (τm(5)U) in the anticodon wobble position of five mitochondrial tRNAs. All case subjects presented with lactic acidosis and nine developed hypertrophic cardiomyopathy. In contrast to individuals with mutations in MTO1, the protein product of which is predicted to participate in the generation of the same modification, most individuals with GTPBP3 mutations developed neurological symptoms and MRI involvement of thalamus, putamen, and brainstem resembling Leigh syndrome. Our study of a mitochondrial translation disorder points toward the importance of posttranscriptional modification of mitochondrial tRNAs for proper mitochondrial function.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
MTO1 mutations are associated with hypertrophic cardiomyopathy and lactic acidosis and cause respiratory chain deficiency in humans and yeast.Hum Mutat. 2013 Nov;34(11):1501-9. doi: 10.1002/humu.22393. Epub 2013 Sep 17. Hum Mutat. 2013. PMID: 23929671 Free PMC article.
-
Defects in the mitochondrial-tRNA modification enzymes MTO1 and GTPBP3 promote different metabolic reprogramming through a HIF-PPARγ-UCP2-AMPK axis.Sci Rep. 2018 Jan 18;8(1):1163. doi: 10.1038/s41598-018-19587-5. Sci Rep. 2018. PMID: 29348686 Free PMC article.
-
Mutations of the mitochondrial-tRNA modifier MTO1 cause hypertrophic cardiomyopathy and lactic acidosis.Am J Hum Genet. 2012 Jun 8;90(6):1079-87. doi: 10.1016/j.ajhg.2012.04.011. Epub 2012 May 17. Am J Hum Genet. 2012. PMID: 22608499 Free PMC article.
-
Leigh syndrome and hypertrophic cardiomyopathy in an infant with a mitochondrial DNA point mutation (T8993G).Am J Med Genet. 1994 Apr 15;50(3):265-71. doi: 10.1002/ajmg.1320500310. Am J Med Genet. 1994. PMID: 8042671 Review.
-
Human mitochondrial diseases caused by lack of taurine modification in mitochondrial tRNAs.Wiley Interdiscip Rev RNA. 2011 May-Jun;2(3):376-86. doi: 10.1002/wrna.65. Epub 2011 Feb 25. Wiley Interdiscip Rev RNA. 2011. PMID: 21957023 Review.
Cited by
-
Mitochondrial transcript maturation and its disorders.J Inherit Metab Dis. 2015 Jul;38(4):655-80. doi: 10.1007/s10545-015-9859-z. Epub 2015 May 28. J Inherit Metab Dis. 2015. PMID: 26016801 Free PMC article. Review.
-
CLIN_SKAT: an R package to conduct association analysis using functionally relevant variants.BMC Bioinformatics. 2022 Oct 23;23(1):441. doi: 10.1186/s12859-022-04987-2. BMC Bioinformatics. 2022. PMID: 36274122 Free PMC article.
-
The expanding world of tRNA modifications and their disease relevance.Nat Rev Mol Cell Biol. 2021 Jun;22(6):375-392. doi: 10.1038/s41580-021-00342-0. Epub 2021 Mar 3. Nat Rev Mol Cell Biol. 2021. PMID: 33658722 Review.
-
Recent topics: the diagnosis, molecular genesis, and treatment of mitochondrial diseases.J Hum Genet. 2019 Feb;64(2):113-125. doi: 10.1038/s10038-018-0528-6. Epub 2018 Nov 21. J Hum Genet. 2019. PMID: 30459337 Review.
-
NSUN2 introduces 5-methylcytosines in mammalian mitochondrial tRNAs.Nucleic Acids Res. 2019 Sep 19;47(16):8720-8733. doi: 10.1093/nar/gkz559. Nucleic Acids Res. 2019. PMID: 31276587 Free PMC article.
References
-
- Osawa S. ASM Press; Washington, DC: 1995. Evolution of the Genetic Code.
-
- Suzuki T. Biosynthesis and function of tRNA wobble modifications. Top. Curr. Genet. 2005;12:23–69.
-
- Colby G., Wu M., Tzagoloff A. MTO1 codes for a mitochondrial protein required for respiration in paromomycin-resistant mutants of Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:27945–27952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

