Bioenergetic dysfunction and inflammation in Alzheimer's disease: a possible connection
- PMID: 25426068
- PMCID: PMC4226164
- DOI: 10.3389/fnagi.2014.00311
Bioenergetic dysfunction and inflammation in Alzheimer's disease: a possible connection
Abstract
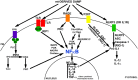
Inflammation is observed in Alzheimer's disease (AD) subject brains. Inflammation-relevant genes are increasingly implicated in AD genetic studies, and inflammatory cytokines to some extent even function as peripheral biomarkers. What underlies AD inflammation is unclear, but no "foreign" agent has been implicated. This suggests that internally produced damage-associated molecular pattern (DAMPs) molecules may drive inflammation in AD. A more complete characterization and understanding of AD-relevant DAMPs could advance our understanding of AD and suggest novel therapeutic strategies. In this review, we consider the possibility that mitochondria, intracellular organelles that resemble bacteria in many ways, trigger and maintain chronic inflammation in AD subjects. Data supporting the possible nexus between AD-associated bioenergetic dysfunction are discussed.
Keywords: Alzheimer’s disease; DAMP; bioenergetics; inflammation; mitochondria.
Figures
Similar articles
-
Mitochondrial lysates induce inflammation and Alzheimer's disease-relevant changes in microglial and neuronal cells.J Alzheimers Dis. 2015;45(1):305-18. doi: 10.3233/JAD-142334. J Alzheimers Dis. 2015. PMID: 25537010 Free PMC article.
-
Mitochondria, Cybrids, Aging, and Alzheimer's Disease.Prog Mol Biol Transl Sci. 2017;146:259-302. doi: 10.1016/bs.pmbts.2016.12.017. Epub 2017 Feb 1. Prog Mol Biol Transl Sci. 2017. PMID: 28253988 Free PMC article. Review.
-
Neuroinflammation in Alzheimer's Disease.Biomedicines. 2021 May 7;9(5):524. doi: 10.3390/biomedicines9050524. Biomedicines. 2021. PMID: 34067173 Free PMC article. Review.
-
Cardiolipin profile changes are associated to the early synaptic mitochondrial dysfunction in Alzheimer's disease.J Alzheimers Dis. 2015;43(4):1375-92. doi: 10.3233/JAD-141002. J Alzheimers Dis. 2015. PMID: 25182746
-
Precursor-Independent Overproduction of Beta-Amyloid in AD: Mitochondrial Dysfunction as Possible Initiator of Asymmetric RNA-Dependent βAPP mRNA Amplification. An Engine that Drives Alzheimer's Disease.Ann Integr Mol Med. 2019;1(1):61-74. Epub 2019 Nov 20. Ann Integr Mol Med. 2019. PMID: 31858090 Free PMC article.
Cited by
-
Exploring the Mechanisms and Therapeutic Approaches of Mitochondrial Dysfunction in Alzheimer's Disease: An Educational Literature Review.Mol Neurobiol. 2024 Sep 10. doi: 10.1007/s12035-024-04468-y. Online ahead of print. Mol Neurobiol. 2024. PMID: 39254911 Review.
-
Neuroinflammatory Mechanisms of Mitochondrial Dysfunction and Neurodegeneration in Glaucoma.J Ophthalmol. 2021 Apr 15;2021:4581909. doi: 10.1155/2021/4581909. eCollection 2021. J Ophthalmol. 2021. PMID: 33953963 Free PMC article. Review.
-
An Integrative Overview of Non-Amyloid and Non-Tau Pathologies in Alzheimer's Disease.Neurochem Res. 2019 Jan;44(1):12-21. doi: 10.1007/s11064-018-2603-y. Epub 2018 Aug 6. Neurochem Res. 2019. PMID: 30084096 Free PMC article. Review.
-
Glaucoma: from pathogenic mechanisms to retinal glial cell response to damage.Front Cell Neurosci. 2024 Jan 25;18:1354569. doi: 10.3389/fncel.2024.1354569. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38333055 Free PMC article. Review.
-
Insulin Resistance, a Risk Factor for Alzheimer's Disease: Pathological Mechanisms and a New Proposal for a Preventive Therapeutic Approach.Biomedicines. 2024 Aug 19;12(8):1888. doi: 10.3390/biomedicines12081888. Biomedicines. 2024. PMID: 39200352 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

