Phase I trial of cyclophosphamide as an immune modulator for optimizing oncolytic reovirus delivery to solid tumors
- PMID: 25424857
- PMCID: PMC4821068
- DOI: 10.1158/1078-0432.CCR-14-1770
Phase I trial of cyclophosphamide as an immune modulator for optimizing oncolytic reovirus delivery to solid tumors
Abstract
Purpose: Reovirus is a wild-type oncolytic virus that is ubiquitous in the environment; most patients are therefore preimmune. Therapeutic administration leads to an increase in neutralizing antireovirus antibody (NARA) titer. We hypothesized that if NARA limited reovirus antitumor activity, the effect might be attenuated by coadministration of cyclophosphamide.
Experimental design: In a phase I study, patients with advanced cancer received cyclophosphamide 3 days before intravenous reovirus serotype 3 Dearing (RT3D). The primary objective was to reduce the resulting rise in NARA titer. Cyclophosphamide dose was escalated from 25-1,000 mg/m(2) through nine cohorts; we aimed to define a well-tolerated immunomodulatory dose.
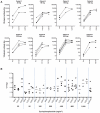
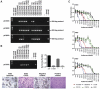
Results: The combination was well tolerated in 36 patients, with grade 3/4 toxicities only seen at or above the maximum tolerated dose of cyclophosphamide, which was 800 mg/m(2) combined with reovirus. Immunosuppressive effect, defined as maintaining NARA titer rise below a predefined threshold, was observed in only one patient. Furthermore, despite expected myelosuppression seen at higher cyclophosphamide doses, no changes in T-cell subsets, including Tregs, occurred with dose escalation. Viable virus was detected in association with peripheral blood mononuclear cells (PBMC) from 14% of patients 10 days after the last RT3D injection, despite high plasma NARA titer, demonstrating a potential mechanism for prolonged evasion of neutralization by reovirus.
Conclusions: Coadministration of cyclophosphamide with reovirus is safe, but does not attenuate host antiviral responses. Alternative immunomodulation approaches should be explored, but association with PBMCs may allow reovirus to persist and evade even high levels of neutralizing antibodies.
©2014 American Association for Cancer Research.
Figures
Similar articles
-
Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial.Gene Ther. 2008 Jun;15(12):911-20. doi: 10.1038/gt.2008.21. Epub 2008 Mar 6. Gene Ther. 2008. PMID: 18323793 Clinical Trial.
-
Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies.Clin Cancer Res. 2012 Apr 1;18(7):2080-9. doi: 10.1158/1078-0432.CCR-11-2181. Epub 2012 Feb 7. Clin Cancer Res. 2012. PMID: 22316603 Free PMC article. Clinical Trial.
-
A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer.Clin Cancer Res. 2008 Nov 1;14(21):7127-37. doi: 10.1158/1078-0432.CCR-08-0524. Clin Cancer Res. 2008. PMID: 18981012 Clinical Trial.
-
Oncolytic viral therapy using reovirus.Methods Mol Biol. 2009;542:607-34. doi: 10.1007/978-1-59745-561-9_31. Methods Mol Biol. 2009. PMID: 19565924 Review.
-
Reovirus-based therapy for cancer.Expert Opin Biol Ther. 2009 Jul;9(7):817-30. doi: 10.1517/14712590903002039. Expert Opin Biol Ther. 2009. PMID: 19527106 Review.
Cited by
-
The oncolytic virus, pelareorep, as a novel anticancer agent: a review.Invest New Drugs. 2015 Jun;33(3):761-74. doi: 10.1007/s10637-015-0216-8. Epub 2015 Feb 19. Invest New Drugs. 2015. PMID: 25693885 Review.
-
Viruses as nanomedicine for cancer.Int J Nanomedicine. 2016 Sep 21;11:4835-4847. doi: 10.2147/IJN.S116447. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27703350 Free PMC article. Review.
-
Comparison between intratumoral and intravenously administered oncolytic virus therapy with Newcastle disease virus in a xenograft murine model for pancreatic adenocarcinoma.Heliyon. 2022 Jul 9;8(7):e09915. doi: 10.1016/j.heliyon.2022.e09915. eCollection 2022 Jul. Heliyon. 2022. PMID: 35874055 Free PMC article.
-
Oncolytic Viruses: An Inventory of Shedding Data from Clinical Trials and Elements for the Environmental Risk Assessment.Vaccines (Basel). 2023 Sep 1;11(9):1448. doi: 10.3390/vaccines11091448. Vaccines (Basel). 2023. PMID: 37766125 Free PMC article. Review.
-
Neutralizing Antibodies Impair the Oncolytic Efficacy of Reovirus but Permit Effective Combination with T cell-Based Immunotherapies.Cancer Immunol Res. 2024 Mar 4;12(3):334-349. doi: 10.1158/2326-6066.CIR-23-0480. Cancer Immunol Res. 2024. PMID: 38194598 Free PMC article.
References
-
- Rosen L, Evans HE, Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. - PubMed
-
- Sabin AB. Reoviruses. A new group of respiratory and enteric viruses formerly classified as ECHO type 10 is described. Science. 1959;130:1387–9. - PubMed
-
- Jackson G, Muldoon R, Cooper R. Reovirus type 1 as an etiologic agent of the common cold. J Clin Invest. 1961;40:1051.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

