The role of phosphoinositide-regulated actin reorganization in chemotaxis and cell migration
- PMID: 25420930
- PMCID: PMC4290701
- DOI: 10.1111/bph.12777
The role of phosphoinositide-regulated actin reorganization in chemotaxis and cell migration
Abstract
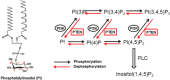
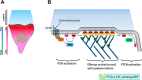
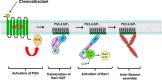
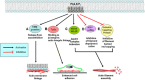
Reorganization of the actin cytoskeleton is essential for cell motility and chemotaxis. Actin-binding proteins (ABPs) and membrane lipids, especially phosphoinositides PI(4,5)P2 and PI(3,4,5)P3 are involved in the regulation of this reorganization. At least 15 ABPs have been reported to interact with, or regulated by phosphoinositides (PIPs) whose synthesis is regulated by extracellular signals. Recent studies have uncovered several parallel intracellular signalling pathways that crosstalk in chemotaxing cells. Here, we review the roles of ABPs and phosphoinositides in chemotaxis and cell migration.
Linked articles: This article is part of a themed section on Cytoskeleton, Extracellular Matrix, Cell Migration, Wound Healing and Related Topics. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-24.
© 2014 The British Pharmacological Society.
Figures
Similar articles
-
Phosphoinositides in the regulation of actin cortex and cell migration.Biochim Biophys Acta. 2015 Jun;1851(6):824-31. doi: 10.1016/j.bbalip.2014.10.011. Epub 2014 Oct 29. Biochim Biophys Acta. 2015. PMID: 25449647 Review.
-
Phosphoinositides in chemotaxis.Subcell Biochem. 2012;59:217-54. doi: 10.1007/978-94-007-3015-1_7. Subcell Biochem. 2012. PMID: 22374092 Review.
-
PI 3-kinases and PTEN: how opposites chemoattract.Cell. 2002 May 31;109(5):541-4. doi: 10.1016/s0092-8674(02)00765-1. Cell. 2002. PMID: 12062096 Review.
-
The regulation of cell motility and chemotaxis by phospholipid signaling.J Cell Sci. 2008 Mar 1;121(Pt 5):551-9. doi: 10.1242/jcs.023333. J Cell Sci. 2008. PMID: 18287584 Free PMC article. Review.
-
Mechanistic principles underlying regulation of the actin cytoskeleton by phosphoinositides.Proc Natl Acad Sci U S A. 2017 Oct 24;114(43):E8977-E8986. doi: 10.1073/pnas.1705032114. Epub 2017 Oct 9. Proc Natl Acad Sci U S A. 2017. PMID: 29073094 Free PMC article.
Cited by
-
Daam2 couples translocation and clustering of Wnt receptor signalosomes through Rac1.J Cell Sci. 2021 Jan 25;134(2):jcs251140. doi: 10.1242/jcs.251140. J Cell Sci. 2021. PMID: 33310913 Free PMC article.
-
Phosphoinositides: Roles in the Development of Microglial-Mediated Neuroinflammation and Neurodegeneration.Front Cell Neurosci. 2021 Mar 26;15:652593. doi: 10.3389/fncel.2021.652593. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33841102 Free PMC article. Review.
-
Dances with Membranes: Breakthroughs from Super-resolution Imaging.Curr Top Membr. 2015;75:59-123. doi: 10.1016/bs.ctm.2015.03.008. Epub 2015 Apr 15. Curr Top Membr. 2015. PMID: 26015281 Free PMC article. Review.
-
Cetylpyridinium chloride (CPC) reduces zebrafish mortality from influenza infection: Super-resolution microscopy reveals CPC interference with multiple protein interactions with phosphatidylinositol 4,5-bisphosphate in immune function.Toxicol Appl Pharmacol. 2022 Apr 1;440:115913. doi: 10.1016/j.taap.2022.115913. Epub 2022 Feb 9. Toxicol Appl Pharmacol. 2022. PMID: 35149080 Free PMC article.
-
Short Lives with Long-Lasting Effects: Filopodia Protrusions in Neuronal Branching Morphogenesis.PLoS Biol. 2015 Sep 3;13(9):e1002241. doi: 10.1371/journal.pbio.1002241. eCollection 2015. PLoS Biol. 2015. PMID: 26334727 Free PMC article.
References
-
- Ahn JS, Jang IS, Kim DI, Cho KA, Park YH, Kim K, et al. Aging-associated increase of gelsolin for apoptosis resistance. Biochem Biophys Res Commun. 2003;312:1335–1341. - PubMed
-
- Aldrich RA, Steinberg AG, Campbell DC. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954;13:133–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

