Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients
- PMID: 25415285
- PMCID: PMC4352951
- DOI: 10.1097/CJI.0000000000000059
Ex vivo-expanded natural killer cells demonstrate robust proliferation in vivo in high-risk relapsed multiple myeloma patients
Abstract
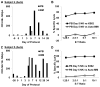
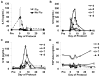
Highly activated/expanded natural killer (NK) cells can be generated by stimulation with the human leukocyte antigen-deficient cell line K562, genetically modified to express 41BB-ligand and membrane-bound interleukin (IL)15. We tested the safety, persistence, and activity of expanded NK cells generated from myeloma patients (auto-NK) or haploidentical family donors (allo-NK) in heavily pretreated patients with high-risk relapsing myeloma. The preparative regimen comprised bortezomib only or bortezomib and immunosuppression with cyclophosphamide, dexamethasone, and fludarabine. NK cells were shipped overnight either cryopreserved or fresh. In 8 patients, up to 1×10⁸ NK cells/kg were infused on day 0 and followed by daily administrations of IL2. Significant in vivo expansion was observed only in the 5 patients receiving fresh products, peaking at or near day 7, with the highest NK-cell counts in 2 subjects who received cells produced in a high concentration of IL2 (500 U/mL). Seven days after infusion, donor NK cells comprised >90% of circulating leukocytes in fresh allo-NK cell recipients, and cytolytic activity against allogeneic myeloma targets was retained in vitro. Among the 7 evaluable patients, there were no serious adverse events that could be related to NK-cell infusion. One patient had a partial response and in another the tempo of disease progression decreased; neither patient required further therapy for 6 months. In the 5 remaining patients, disease progression was not affected by NK-cell infusion. In conclusion, infusion of large numbers of expanded NK cells was feasible and safe; infusing fresh cells was critical to their expansion in vivo.
Conflict of interest statement
There are no relevant conflicts of interest to disclose.
Figures





Similar articles
-
Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation.Br J Haematol. 2008 Dec;143(5):641-53. doi: 10.1111/j.1365-2141.2008.07340.x. Epub 2008 Oct 16. Br J Haematol. 2008. PMID: 18950462 Free PMC article.
-
Haploidentical Natural Killer Cells Infused before Allogeneic Stem Cell Transplantation for Myeloid Malignancies: A Phase I Trial.Biol Blood Marrow Transplant. 2016 Jul;22(7):1290-1298. doi: 10.1016/j.bbmt.2016.04.009. Epub 2016 Apr 16. Biol Blood Marrow Transplant. 2016. PMID: 27090958 Free PMC article. Clinical Trial.
-
A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer.Cytotherapy. 2011 Jan;13(1):98-107. doi: 10.3109/14653249.2010.515582. Epub 2010 Sep 20. Cytotherapy. 2011. PMID: 20849361 Free PMC article. Clinical Trial.
-
NK cell therapy in relapsed refractory multiple myeloma.Clin Immunol. 2023 Jan;246:109168. doi: 10.1016/j.clim.2022.109168. Epub 2022 Oct 29. Clin Immunol. 2023. PMID: 36415020 Review.
-
NK-92: an 'off-the-shelf therapeutic' for adoptive natural killer cell-based cancer immunotherapy.Cancer Immunol Immunother. 2016 Apr;65(4):485-92. doi: 10.1007/s00262-015-1761-x. Epub 2015 Nov 11. Cancer Immunol Immunother. 2016. PMID: 26559813 Free PMC article. Review.
Cited by
-
Adoptive NK Cell Therapy - a Beacon of Hope in Multiple Myeloma Treatment.Front Oncol. 2023 Nov 3;13:1275076. doi: 10.3389/fonc.2023.1275076. eCollection 2023. Front Oncol. 2023. PMID: 38023191 Free PMC article. Review.
-
Analysis of ex vivo expanded and activated clinical-grade human NK cells after cryopreservation.Cytotherapy. 2020 Aug;22(8):450-457. doi: 10.1016/j.jcyt.2020.05.001. Epub 2020 Jun 11. Cytotherapy. 2020. PMID: 32536506 Free PMC article.
-
The Rise of Allogeneic Natural Killer Cells As a Platform for Cancer Immunotherapy: Recent Innovations and Future Developments.Front Immunol. 2017 May 31;8:631. doi: 10.3389/fimmu.2017.00631. eCollection 2017. Front Immunol. 2017. PMID: 28620386 Free PMC article. Review.
-
The application, safety, and future of ex vivo immune cell therapies and prognosis in different malignancies.Bioimpacts. 2023;13(6):439-455. doi: 10.34172/bi.2023.27521. Epub 2023 Jul 29. Bioimpacts. 2023. PMID: 38022382 Free PMC article. Review.
-
Targeting of CXCR3 improves anti-myeloma efficacy of adoptively transferred activated natural killer cells.J Immunother Cancer. 2019 Nov 7;7(1):290. doi: 10.1186/s40425-019-0751-5. J Immunother Cancer. 2019. PMID: 31699153 Free PMC article.
References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. - PubMed
-
- Shaughnessy JD, Jr, Zhan F, Burington BE, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. - PubMed
-
- Carbone E, Neri P, Mesuraca M, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–258. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

