Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors
- PMID: 25410856
- PMCID: PMC4300752
- DOI: 10.1128/JVI.02950-14
Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors
Abstract
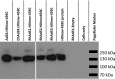
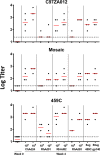
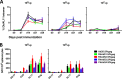
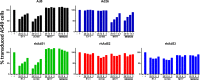
Adenovirus vectors are widely used as vaccine candidates for a variety of pathogens, including HIV-1. To date, human and chimpanzee adenoviruses have been explored in detail as vaccine vectors. The phylogeny of human and chimpanzee adenoviruses is overlapping, and preexisting humoral and cellular immunity to both are exhibited in human populations worldwide. More distantly related adenoviruses may therefore offer advantages as vaccine vectors. Here we describe the primary isolation and vectorization of three novel adenoviruses from rhesus monkeys. The seroprevalence of these novel rhesus monkey adenovirus vectors was extremely low in sub-Saharan Africa human populations, and these vectors proved to have immunogenicity comparable to that of human and chimpanzee adenovirus vaccine vectors in mice. These rhesus monkey adenoviruses phylogenetically clustered with the poorly described adenovirus species G and robustly stimulated innate immune responses. These novel adenoviruses represent a new class of candidate vaccine vectors.
Importance: Although there have been substantial efforts in the development of vaccine vectors from human and chimpanzee adenoviruses, far less is known about rhesus monkey adenoviruses. In this report, we describe the isolation and vectorization of three novel rhesus monkey adenoviruses. These vectors exhibit virologic and immunologic characteristics that make them attractive as potential candidate vaccine vectors for both HIV-1 and other pathogens.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Adenoviruses isolated from wild gorillas are closely related to human species C viruses.Virology. 2013 Sep;444(1-2):119-23. doi: 10.1016/j.virol.2013.05.041. Epub 2013 Jun 24. Virology. 2013. PMID: 23806387
-
Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D.J Virol. 2007 May;81(9):4654-63. doi: 10.1128/JVI.02696-06. Epub 2007 Feb 28. J Virol. 2007. PMID: 17329340 Free PMC article.
-
Rapid Cloning of Novel Rhesus Adenoviral Vaccine Vectors.J Virol. 2018 Feb 26;92(6):e01924-17. doi: 10.1128/JVI.01924-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298888 Free PMC article.
-
Adenoviruses as vaccine vectors.Mol Ther. 2004 Oct;10(4):616-29. doi: 10.1016/j.ymthe.2004.07.013. Mol Ther. 2004. PMID: 15451446 Free PMC article. Review.
-
Viral vectors as vaccine carriers.Curr Opin Virol. 2016 Dec;21:1-8. doi: 10.1016/j.coviro.2016.06.001. Epub 2016 Jun 18. Curr Opin Virol. 2016. PMID: 27327517 Review.
Cited by
-
Identification of a novel neutralization epitope in rhesus AAVs.Mol Ther Methods Clin Dev. 2024 Oct 4;32(4):101350. doi: 10.1016/j.omtm.2024.101350. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39469420 Free PMC article.
-
Development of Zika Virus Vaccines.Vaccines (Basel). 2018 Jan 18;6(1):7. doi: 10.3390/vaccines6010007. Vaccines (Basel). 2018. PMID: 29346287 Free PMC article. Review.
-
Vaccination strategies against Zika virus.Curr Opin Virol. 2017 Apr;23:59-67. doi: 10.1016/j.coviro.2017.03.006. Epub 2017 Apr 19. Curr Opin Virol. 2017. PMID: 28432975 Free PMC article. Review.
-
Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys.Science. 2016 Sep 9;353(6304):1129-32. doi: 10.1126/science.aah6157. Epub 2016 Aug 4. Science. 2016. PMID: 27492477 Free PMC article.
-
Simian adenoviruses as vaccine vectors.Future Virol. 2016 Sep;11(9):649-659. doi: 10.2217/fvl-2016-0070. Epub 2016 Sep 15. Future Virol. 2016. PMID: 29527232 Free PMC article. Review.
References
-
- Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, Johnson JA, Kleinjan J, Yanosick KE, Perry J, Zablowsky E, Abbink P, Peter L, Iampietro MJ, Cheung A, Pau MG, Weijtens M, Goudsmit J, Swann E, Wolff M, Loblein H, Dolin R, Barouch DH. 2013. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 207:240–247. doi:10.1093/infdis/jis670. - DOI - PMC - PubMed
-
- Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93. doi:10.1038/nature10766. - DOI - PMC - PubMed
-
- Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, Dugast AS, Alter G, Ferguson M, Li W, Earl PL, Moss B, Giorgi EE, Szinger JJ, Eller LA, Billings EA, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Pau MG, Schuitemaker H, Robb ML, Kim J H, Korber BT, Michael NL. 2013. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155:531–539. doi:10.1016/j.cell.2013.09.061. - DOI - PMC - PubMed
-
- Hosseini SY, Sabahi F, Moazzeni SM, Modarressi MH, Saberi Firoozi M, Ravanshad M. 2012. Construction and preparation of three recombinant adenoviruses expressing truncated NS3 and core genes of hepatitis C virus for vaccine purposes. Hepat Mon 12:e6130. doi:10.5812/hepatmon.6130. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

