Cystic fibrosis genetics: from molecular understanding to clinical application
- PMID: 25404111
- PMCID: PMC4364438
- DOI: 10.1038/nrg3849
Cystic fibrosis genetics: from molecular understanding to clinical application
Abstract
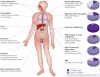
The availability of the human genome sequence and tools for interrogating individual genomes provide an unprecedented opportunity to apply genetics to medicine. Mendelian conditions, which are caused by dysfunction of a single gene, offer powerful examples that illustrate how genetics can provide insights into disease. Cystic fibrosis, one of the more common lethal autosomal recessive Mendelian disorders, is presented here as an example. Recent progress in elucidating disease mechanism and causes of phenotypic variation, as well as in the development of treatments, demonstrates that genetics continues to play an important part in cystic fibrosis research 25 years after the discovery of the disease-causing gene.
Figures

Similar articles
-
[Modifier genes and cystic fibrosis].Arch Pediatr. 2006 Jan;13(1):57-63. doi: 10.1016/j.arcped.2005.09.029. Epub 2005 Nov 7. Arch Pediatr. 2006. PMID: 16274977 Review. French.
-
Molecular Diagnosis of Cystic Fibrosis.Curr Protoc Hum Genet. 2016 Jan 1;88:9.28.1-9.28.6. doi: 10.1002/0471142905.hg0928s88. Curr Protoc Hum Genet. 2016. PMID: 26724724
-
Precision Genomic Medicine in Cystic Fibrosis.Clin Transl Sci. 2015 Oct;8(5):606-10. doi: 10.1111/cts.12292. Epub 2015 Jun 15. Clin Transl Sci. 2015. PMID: 26073768 Free PMC article. Review.
-
First DNA-based test to detect cystic fibrosis.FDA Consum. 2005 Jul-Aug;39(4):4. FDA Consum. 2005. PMID: 16252390 No abstract available.
-
Molecular diagnosis of cystic fibrosis.Expert Rev Mol Diagn. 2002 May;2(3):240-56. doi: 10.1586/14737159.2.3.240. Expert Rev Mol Diagn. 2002. PMID: 12050863 Review.
Cited by
-
Deep representation learning of electronic health records to unlock patient stratification at scale.NPJ Digit Med. 2020 Jul 17;3:96. doi: 10.1038/s41746-020-0301-z. eCollection 2020. NPJ Digit Med. 2020. PMID: 32699826 Free PMC article.
-
Autophagy in Pulmonary Diseases.Am J Respir Crit Care Med. 2016 Nov 15;194(10):1196-1207. doi: 10.1164/rccm.201512-2468SO. Am J Respir Crit Care Med. 2016. PMID: 27579514 Free PMC article. Review.
-
Chronic rhinosinusitis in patients with cystic fibrosis-Current management and new treatments.Laryngoscope Investig Otolaryngol. 2020 Jun 13;5(3):368-374. doi: 10.1002/lio2.401. eCollection 2020 Jun. Laryngoscope Investig Otolaryngol. 2020. PMID: 32596478 Free PMC article. Review.
-
Influenza, SARS-CoV-2, and Their Impact on Chronic Lung Diseases and Fibrosis: Exploring Therapeutic Options.Am J Pathol. 2024 Oct;194(10):1807-1822. doi: 10.1016/j.ajpath.2024.06.004. Epub 2024 Jul 18. Am J Pathol. 2024. PMID: 39032604 Review.
-
Antibacterial Activity of a Natural Clay Mineral against Burkholderia cepacia Complex and Other Bacterial Pathogens Isolated from People with Cystic Fibrosis.Microorganisms. 2023 Jan 6;11(1):150. doi: 10.3390/microorganisms11010150. Microorganisms. 2023. PMID: 36677442 Free PMC article.
References
-
- Cystic Fibrosis Foundation . Cystic Fibrosis Foundation Patient Registry Annual Data Report 2011. Cystic Fibrosis Foundation; 2012.
-
- Rommens JM, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. - PubMed
-
- Riordan JR, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. - PubMed
-
- Kerem B, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. References 3-5 are landmark papers from 25 years ago reporting the discovery of the CFTR gene. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

