Mice lacking Axl and Mer tyrosine kinase receptors are susceptible to experimental autoimmune orchitis induction
- PMID: 25403570
- PMCID: PMC5800791
- DOI: 10.1038/icb.2014.97
Mice lacking Axl and Mer tyrosine kinase receptors are susceptible to experimental autoimmune orchitis induction
Abstract
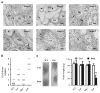
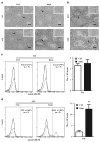
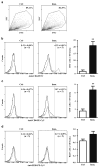
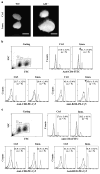
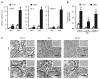
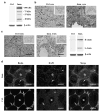
The mammalian testis is an immunoprivileged organ where male germ cell autoantigens are immunologically ignored. Both systemic immune tolerance to autoantigens and local immunosuppressive milieu contribute to the testicular immune privilege. Testicular immunosuppression has been intensively studied, but information on systemic immune tolerance to autoantigens is lacking. In the present study, we aimed to determine the role of Axl and Mer receptor tyrosine kinases in maintaining the systemic tolerance to male germ cell antigens using the experimental autoimmune orchitis (EAO) model. Axl and Mer double-knockout (Axl(-/-)Mer(-/-)) mice developed evident EAO after a single immunization with germ cell homogenates emulsified with complete Freund's adjuvant. EAO was characterized by the accumulation of macrophages and T lymphocytes in the testis. Damage to the seminiferous epithelium was also observed. EAO induction was associated with pro-inflammatory cytokine upregulation in the testes, impaired permeability of the blood-testis barrier and generation of autoantibodies against germ cell antigens in Axl(-/-)Mer(-/-) mice. Immunization also induced mild EAO in Axl or Mer single-gene-knockout mice. By contrast, a single immunization failed to induce EAO in wild-type mice. The results indicate that Axl and Mer receptors cooperatively regulate the systemic immune tolerance to male germ cell antigens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Roles of Toll-like receptors 2 and 4 in mediating experimental autoimmune orchitis induction in mice.Biol Reprod. 2015 Mar;92(3):63. doi: 10.1095/biolreprod.114.123901. Epub 2015 Jan 14. Biol Reprod. 2015. PMID: 25588509 Free PMC article.
-
Breakdown of immune homeostasis in the testis of mice lacking Tyro3, Axl and Mer receptor tyrosine kinases.Immunol Cell Biol. 2013 Jul;91(6):416-26. doi: 10.1038/icb.2013.22. Epub 2013 May 21. Immunol Cell Biol. 2013. PMID: 23689306
-
Experimental model of autoimmune orchitis with abdominal placement of donor's testes, epididymides, and vasa deferentia in recipient mice.J Reprod Immunol. 2011 Aug;90(2):195-201. doi: 10.1016/j.jri.2011.03.008. Epub 2011 Jun 30. J Reprod Immunol. 2011. PMID: 21722965
-
Role of testicular autoantigens and influence of lymphokines in testicular autoimmune disease.J Reprod Immunol. 1990 Aug;18(1):89-103. doi: 10.1016/0165-0378(90)90026-3. J Reprod Immunol. 1990. PMID: 2213733 Review.
-
Pathomechanisms of Autoimmune Based Testicular Inflammation.Front Immunol. 2020 Sep 25;11:583135. doi: 10.3389/fimmu.2020.583135. eCollection 2020. Front Immunol. 2020. PMID: 33101310 Free PMC article. Review.
Cited by
-
Microcystin-Leucine Arginine Causes Cytotoxic Effects in Sertoli Cells Resulting in Reproductive Dysfunction in Male Mice.Sci Rep. 2016 Dec 15;6:39238. doi: 10.1038/srep39238. Sci Rep. 2016. PMID: 27976743 Free PMC article.
-
Roles of Toll-like receptors 2 and 4 in mediating experimental autoimmune orchitis induction in mice.Biol Reprod. 2015 Mar;92(3):63. doi: 10.1095/biolreprod.114.123901. Epub 2015 Jan 14. Biol Reprod. 2015. PMID: 25588509 Free PMC article.
-
Immunologic Environment of the Testis.Adv Exp Med Biol. 2021;1288:49-67. doi: 10.1007/978-3-030-77779-1_3. Adv Exp Med Biol. 2021. PMID: 34453731
-
Giving AXL the axe: targeting AXL in human malignancy.Br J Cancer. 2017 Feb 14;116(4):415-423. doi: 10.1038/bjc.2016.428. Epub 2017 Jan 10. Br J Cancer. 2017. PMID: 28072762 Free PMC article. Review.
-
Rethinking Phagocytes: Clues from the Retina and Testes.Trends Cell Biol. 2018 Apr;28(4):317-327. doi: 10.1016/j.tcb.2018.01.004. Epub 2018 Feb 14. Trends Cell Biol. 2018. PMID: 29454661 Free PMC article. Review.
References
-
- Yule TD, Montoya GD, Russell LD, Williams TM, Tung KS. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol. 1988;141:1161–1167. - PubMed
-
- Jacobo P, Guazzone VA, Theas MS, Lustig L. Testicular autoimmunity. Autoimmun Rev. 2011;10:201–204. - PubMed
-
- Naito M, Terayama H, Hirai S, Qu N, Lustig L, Itoh M. Experimental autoimmune orchitis as a model of immunological male infertility. Med Mol Morphol. 2012;45:185–189. - PubMed
-
- Rival C, Theas MS, Suescun MO, Jacobo P, Guazzone V, van Rooijen N, et al. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J Pathol. 2008;215:108–117. - PubMed
-
- Jacobo P, Guazzone VA, Jarazo-Dietrich S, Theas MS, Lustig L. Differential changes in CD4+ and CD8+ effector and regulatory T lymphocyte subsets in the testis of rats undergoing autoimmune orchitis. J Reprod Immunol. 2009;81:44–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

