A histological evaluation and in vivo assessment of intratumoral near infrared photothermal nanotherapy-induced tumor regression
- PMID: 25395847
- PMCID: PMC4227627
- DOI: 10.2147/IJN.S60648
A histological evaluation and in vivo assessment of intratumoral near infrared photothermal nanotherapy-induced tumor regression
Abstract
Purpose: Nanoparticle (NP)-enabled near infrared (NIR) photothermal therapy has realized limited success in in vivo studies as a potential localized cancer therapy. This is primarily due to a lack of successful methods that can prevent NP uptake by the reticuloendothelial system, especially the liver and kidney, and deliver sufficient quantities of intravenously injected NPs to the tumor site. Histological evaluation of photothermal therapy-induced tumor regression is also neglected in the current literature. This report demonstrates and histologically evaluates the in vivo potential of NIR photothermal therapy by circumventing the challenges of intravenous NP delivery and tumor targeting found in other photothermal therapy studies.
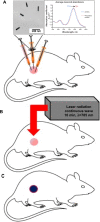
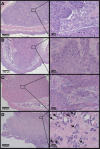
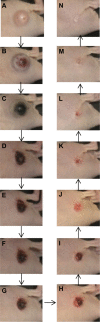
Methods: Subcutaneous Cal 27 squamous cell carcinoma xenografts received photothermal nanotherapy treatments, radial injections of polyethylene glycol (PEG)-ylated gold nanorods and one NIR 785 nm laser irradiation for 10 minutes at 9.5 W/cm(2). Tumor response was measured for 10-15 days, gross changes in tumor size were evaluated, and the remaining tumors or scar tissues were excised and histologically analyzed.
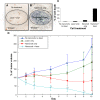
Results: The single treatment of intratumoral nanorod injections followed by a 10 minute NIR laser treatment also known as photothermal nanotherapy, resulted in ~100% tumor regression in ~90% of treated tumors, which was statistically significant in a comparison to the average of all three control groups over time (P<0.01).
Conclusion: Photothermal nanotherapy, or intratumoral nanorod injections followed by NIR laser irradiation of tumors and tumor margins, demonstrate the potential of NIR photothermal therapy as a viable localized treatment approach for primary and early stage tumors, and prevents NP uptake by the reticuloendothelial system.
Keywords: PEGylation; cancer treatment; gold nanorods; intratumoral; laser therapy; malignancy; nanoparticles; photothermal cancer therapy.
Figures
Similar articles
-
Evaluation of a nanocomposite of PEG-curcumin-gold nanoparticles as a near-infrared photothermal agent: an in vitro and animal model investigation.Lasers Med Sci. 2018 Nov;33(8):1769-1779. doi: 10.1007/s10103-018-2538-1. Epub 2018 May 22. Lasers Med Sci. 2018. PMID: 29790012
-
Polysarcosine brush stabilized gold nanorods for in vivo near-infrared photothermal tumor therapy.Acta Biomater. 2017 Mar 1;50:534-545. doi: 10.1016/j.actbio.2016.12.050. Epub 2016 Dec 25. Acta Biomater. 2017. PMID: 28027959
-
Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice.Proc Natl Acad Sci U S A. 2017 Apr 11;114(15):E3110-E3118. doi: 10.1073/pnas.1619302114. Epub 2017 Mar 29. Proc Natl Acad Sci U S A. 2017. PMID: 28356516 Free PMC article.
-
[Remodeling of tumor stroma combined with photothermal therapy in the treatment of triple-negative breast cancer].Zhonghua Zhong Liu Za Zhi. 2023 Nov 23;45(11):926-933. doi: 10.3760/cma.j.cn12152-20221108-00747. Zhonghua Zhong Liu Za Zhi. 2023. PMID: 37968077 Chinese.
-
The impact of size and surface ligand of gold nanorods on liver cancer accumulation and photothermal therapy in the second near-infrared window.J Colloid Interface Sci. 2020 Apr 1;565:186-196. doi: 10.1016/j.jcis.2020.01.026. Epub 2020 Jan 13. J Colloid Interface Sci. 2020. PMID: 31972332
Cited by
-
Visible to near-infrared refractive properties of freshly-excised human-liver tissues: marking hepatic malignancies.Sci Rep. 2016 Jun 14;6:27910. doi: 10.1038/srep27910. Sci Rep. 2016. PMID: 27297034 Free PMC article.
-
Gold nanorods/mesoporous silica-based nanocomposite as theranostic agents for targeting near-infrared imaging and photothermal therapy induced with laser.Int J Nanomedicine. 2015 Jul 28;10:4747-61. doi: 10.2147/IJN.S82940. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26251596 Free PMC article.
-
Photothermal ablation of bone metastasis of breast cancer using PEGylated multi-walled carbon nanotubes.Sci Rep. 2015 Jun 30;5:11709. doi: 10.1038/srep11709. Sci Rep. 2015. PMID: 26122018 Free PMC article.
-
Magnetic Targeting of Magneto-Plasmonic Nanoparticles and Their Effects on Temperature Profile of NIR Laser Irradiated to CT26 Tumor in BALB/C Mice.J Biomed Phys Eng. 2021 Jun 1;11(3):281-288. doi: 10.31661/jbpe.v0i0.1032. eCollection 2021 Jun. J Biomed Phys Eng. 2021. PMID: 34189116 Free PMC article.
-
Effects of arginine-glycine-aspartic acid peptide-conjugated quantum dots-induced photodynamic therapy on pancreatic carcinoma in vivo.Int J Nanomedicine. 2017 Apr 5;12:2769-2779. doi: 10.2147/IJN.S130799. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28435257 Free PMC article.
References
-
- Zhao Z, Wu F. Minimally-invasive thermal ablation of early-stage breast cancer: a systemic review. Eur J Surg Oncol. 2010;36(12):1149–1155. - PubMed
-
- Hall-Craggs MA, Vaidya JS. Minimally invasive therapy for the treatment of breast tumours. Eur J Radiol. 2002;42(1):52–57. - PubMed
-
- Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17(1):171–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

