Targeting the INCENP IN-box-Aurora B interaction to inhibit CPC activity in vivo
- PMID: 25392451
- PMCID: PMC4248066
- DOI: 10.1098/rsob.140163
Targeting the INCENP IN-box-Aurora B interaction to inhibit CPC activity in vivo
Abstract
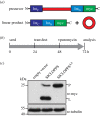
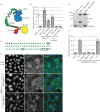
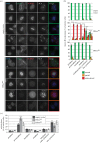
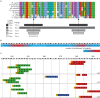
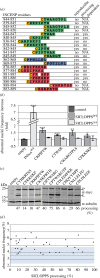
The chromosome passenger complex (CPC) is an essential regulator of mitosis and cytokinesis. The CPC consists of Aurora B kinase, inner centromere protein (INCENP), and the targeting subunits survivin and borealin/Dasra B. INCENP is a scaffolding subunit for the CPC and activates Aurora B via its conserved IN-box domain. We show that overexpression of soluble IN-box in HeLa cells affects endogenous CPC localization and produces a significant increase in multinucleated and micronucleated cells consistent with CPC loss of function. The dominant-negative effect of soluble IN-box expression depends on residues corresponding to hINCENP W845 and/or F881, suggesting that these are essential for Aurora B binding in vivo. We then screened a targeted library of small (five to nine residues long) circular peptide (CP) IN-box fragments generated using split intein circular ligation of proteins and peptides (SICLOPPS) methodology. We identified a number of CPs that caused modest but reproducible increases in rates of multinucleated and micronucleated cells. Our results provide proof of concept that inhibition of the Aurora B-IN-box interaction is a viable strategy for interfering with CPC function in vivo.
Keywords: Aurora B; INCENP; chromosomal passenger complex; cyclic peptide; cytokinesis; mitosis.
Figures
Similar articles
-
Aurora-C Interactions with Survivin and INCENP Reveal Shared and Distinct Features Compared with Aurora-B Chromosome Passenger Protein Complex.PLoS One. 2016 Jun 22;11(6):e0157305. doi: 10.1371/journal.pone.0157305. eCollection 2016. PLoS One. 2016. PMID: 27332895 Free PMC article.
-
The Inner Centromere Protein (INCENP) Coil Is a Single α-Helix (SAH) Domain That Binds Directly to Microtubules and Is Important for Chromosome Passenger Complex (CPC) Localization and Function in Mitosis.J Biol Chem. 2015 Aug 28;290(35):21460-72. doi: 10.1074/jbc.M115.645317. Epub 2015 Jul 14. J Biol Chem. 2015. PMID: 26175154 Free PMC article.
-
Aurora B kinase activity-dependent and -independent functions of the chromosomal passenger complex in regulating sister chromatid cohesion.J Biol Chem. 2019 Feb 8;294(6):2021-2035. doi: 10.1074/jbc.RA118.005978. Epub 2018 Dec 6. J Biol Chem. 2019. PMID: 30523151 Free PMC article.
-
The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis.Nat Rev Mol Cell Biol. 2012 Dec;13(12):789-803. doi: 10.1038/nrm3474. Nat Rev Mol Cell Biol. 2012. PMID: 23175282 Free PMC article. Review.
-
Chromosomal passengers: conducting cell division.Nat Rev Mol Cell Biol. 2007 Oct;8(10):798-812. doi: 10.1038/nrm2257. Nat Rev Mol Cell Biol. 2007. PMID: 17848966 Review.
Cited by
-
Functional high-throughput screening reveals miR-323a-5p and miR-342-5p as new tumor-suppressive microRNA for neuroblastoma.Cell Mol Life Sci. 2019 Jun;76(11):2231-2243. doi: 10.1007/s00018-019-03041-4. Epub 2019 Feb 15. Cell Mol Life Sci. 2019. PMID: 30770954 Free PMC article.
-
Aurora-A Kinase as a Promising Therapeutic Target in Cancer.Front Oncol. 2016 Jan 6;5:295. doi: 10.3389/fonc.2015.00295. eCollection 2015. Front Oncol. 2016. PMID: 26779440 Free PMC article. Review.
-
Minimal model for collective kinetochore-microtubule dynamics.Proc Natl Acad Sci U S A. 2015 Oct 13;112(41):12699-704. doi: 10.1073/pnas.1513512112. Epub 2015 Sep 28. Proc Natl Acad Sci U S A. 2015. PMID: 26417109 Free PMC article.
-
AURKB Promotes the Metastasis of Gastric Cancer, Possibly by Inducing EMT.Cancer Manag Res. 2020 Aug 5;12:6947-6958. doi: 10.2147/CMAR.S254250. eCollection 2020. Cancer Manag Res. 2020. PMID: 32801915 Free PMC article.
-
Head-to-tail cyclization of side chain-protected linear peptides to recapitulate genetically-encoded cyclized peptides.Pept Sci (Hoboken). 2022 May;114(3):e24254. doi: 10.1002/pep2.24254. Epub 2022 Jan 6. Pept Sci (Hoboken). 2022. PMID: 35864841 Free PMC article.
References
-
- Cooke CA, Heck MM, Earnshaw WC. 1987. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J. Cell Biol. 105, 2053–2067. (doi:10.1083/jcb.105.5.2053) - DOI - PMC - PubMed
-
- Wheatley SP, Carvalho A, Vagnarelli P, Earnshaw WC. 2001. INCENP is required for proper targeting of Survivin to the centromeres and the anaphase spindle during mitosis. Curr. Biol. 11, 886–890. (doi:10.1016/S0960-9822(01)00238-X) - DOI - PubMed
-
- Bolton MA, Lan W, Powers SE, McCleland ML, Kuang J, Stukenberg PT. 2002. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol. Biol. Cell 13, 3064–3077. (doi:10.1091/mbc.E02-02-0092) - DOI - PMC - PubMed
-
- Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC. 2004. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J. Cell Biol. 166, 179–191. (doi:10.1083/jcb.200404001) - DOI - PMC - PubMed
-
- Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. 2004. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 118, 187–202. (doi:10.1016/j.cell.2004.06.026) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

