MEK1 transduces the prion protein N2 fragment antioxidant effects
- PMID: 25391659
- PMCID: PMC11114014
- DOI: 10.1007/s00018-014-1777-y
MEK1 transduces the prion protein N2 fragment antioxidant effects
Abstract
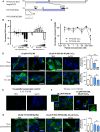
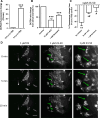
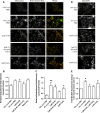
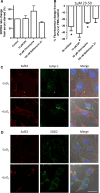
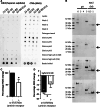
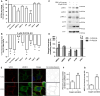
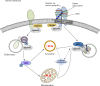
The prion protein (PrP(C)) when mis-folded is causally linked with a group of fatal neurodegenerative diseases called transmissible spongiform encephalopathies or prion diseases. PrP(C) normal function is still incompletely defined with such investigations complicated by PrP(C) post-translational modifications, such as internal cleavage, which feasibly could change, activate, or deactivate the function of this protein. Oxidative stress induces β-cleavage and the N-terminal product of this cleavage event, N2, demonstrates a cellular protective response against oxidative stress. The mechanisms by which N2 mediates cellular antioxidant protection were investigated within an in vitro cell model. N2 protection was regulated by copper binding to the octarepeat domain, directing the route of internalisation, which stimulated MEK1 signalling. Precise membrane interactions of N2, determined by copper saturation, and involving both the copper-co-ordinating octarepeat region and the structure conferred upon the N-terminal polybasic region by the proline motif, were essential for the correct engagement of this pathway. The phenomenon of PrP(C) post-translational modification, such as cleavage and copper co-ordination, as a molecular "switch" for activation or deactivation of certain functions provides new insight into the apparent multi-functionality of PrP(C).
Figures
Similar articles
-
Pathogenic prions deviate PrP(C) signaling in neuronal cells and impair A-beta clearance.Cell Death Dis. 2013 Jan 10;4(1):e456. doi: 10.1038/cddis.2012.195. Cell Death Dis. 2013. PMID: 23303130 Free PMC article.
-
Dominant roles of the polybasic proline motif and copper in the PrP23-89-mediated stress protection response.J Cell Sci. 2009 May 15;122(Pt 10):1518-28. doi: 10.1242/jcs.043604. Epub 2009 Apr 21. J Cell Sci. 2009. PMID: 19383722
-
Understanding the neurospecificity of Prion protein signaling.Front Biosci (Landmark Ed). 2011 Jan 1;16(1):169-86. doi: 10.2741/3682. Front Biosci (Landmark Ed). 2011. PMID: 21196165 Review.
-
The octarepeat region of prion protein, but not the TM1 domain, is important for the antioxidant effect of prion protein.Free Radic Biol Med. 2008 Dec 15;45(12):1622-30. doi: 10.1016/j.freeradbiomed.2008.08.024. Epub 2008 Sep 9. Free Radic Biol Med. 2008. PMID: 18824094
-
The prion protein and lipid rafts.Mol Membr Biol. 2006 Jan-Feb;23(1):89-99. doi: 10.1080/09687860500449994. Mol Membr Biol. 2006. PMID: 16611584 Review.
Cited by
-
Reduced SOD2 expression does not influence prion disease course or pathology in mice.PLoS One. 2021 Nov 4;16(11):e0259597. doi: 10.1371/journal.pone.0259597. eCollection 2021. PLoS One. 2021. PMID: 34735539 Free PMC article.
-
Anchorless risk or released benefit? An updated view on the ADAM10-mediated shedding of the prion protein.Cell Tissue Res. 2023 Apr;392(1):215-234. doi: 10.1007/s00441-022-03582-4. Epub 2022 Jan 27. Cell Tissue Res. 2023. PMID: 35084572 Free PMC article. Review.
-
Prion Protein: The Molecule of Many Forms and Faces.Int J Mol Sci. 2022 Jan 22;23(3):1232. doi: 10.3390/ijms23031232. Int J Mol Sci. 2022. PMID: 35163156 Free PMC article. Review.
-
Biological Functions of the Intrinsically Disordered N-Terminal Domain of the Prion Protein: A Possible Role of Liquid-Liquid Phase Separation.Biomolecules. 2021 Aug 12;11(8):1201. doi: 10.3390/biom11081201. Biomolecules. 2021. PMID: 34439867 Free PMC article. Review.
-
The Role of Cellular Prion Protein in Promoting Stemness and Differentiation in Cancer.Cancers (Basel). 2021 Jan 6;13(2):170. doi: 10.3390/cancers13020170. Cancers (Basel). 2021. PMID: 33418999 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

