NK cells with KIR2DS2 immunogenotype have a functional activation advantage to efficiently kill glioblastoma and prolong animal survival
- PMID: 25381437
- PMCID: PMC4259203
- DOI: 10.4049/jimmunol.1400859
NK cells with KIR2DS2 immunogenotype have a functional activation advantage to efficiently kill glioblastoma and prolong animal survival
Abstract
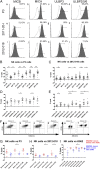
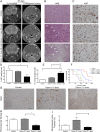
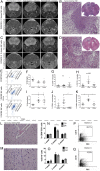
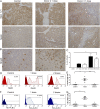
Glioblastomas (GBMs) are lethal brain cancers that are resistant to current therapies. We investigated the cytotoxicity of human allogeneic NK cells against patient-derived GBM in vitro and in vivo, as well as mechanisms mediating their efficacy. We demonstrate that KIR2DS2 immunogenotype NK cells were more potent killers, notwithstanding the absence of inhibitory killer Ig-like receptor (KIR)-HLA ligand mismatch. FACS-sorted and enriched KIR2DS2(+) NK cell subpopulations retained significantly high levels of CD69 and CD16 when in contact with GBM cells at a 1:1 ratio and highly expressed CD107a and secreted more soluble CD137 and granzyme A. In contrast, KIR2DS2(-) immunogenotype donor NK cells were less cytotoxic against GBM and K562, and, similar to FACS-sorted or gated KIR2DS2(-) NK cells, significantly diminished CD16, CD107a, granzyme A, and CD69 when in contact with GBM cells. Furthermore, NK cell-mediated GBM killing in vitro depended upon the expression of ligands for the activating receptor NKG2D and was partially abrogated by Ab blockade. Treatment of GBM xenografts in NOD/SCID mice with NK cells from a KIR2DS2(+) donor lacking inhibitory KIR-HLA ligand mismatch significantly prolonged the median survival to 163 d compared with vehicle controls (log-rank test, p = 0.0001), in contrast to 117.5 d (log-rank test, p = 0.0005) for NK cells with several inhibitory KIR-HLA ligand mismatches but lacking KIR2DS2 genotype. Significantly more CD56(+)CD16(+) NK cells from a KIR2DS2(+) donor survived in nontumor-bearing brains 3 wk after infusion compared with KIR2DS2(-) NK cells, independent of their proliferative capacity. In conclusion, KIR2DS2 identifies potent alloreactive NK cells against GBM that are mediated by commensurate, but dominant, activating signals.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures

Similar articles
-
[Expression of NKG2D ligands in multidrug-resistant nasopharyngeal carcinoma cell line CNE2/DDP and their effects on cytotoxicity of natural killer cells].Nan Fang Yi Ke Da Xue Xue Bao. 2007 Jun;27(6):887-9. Nan Fang Yi Ke Da Xue Xue Bao. 2007. PMID: 17584663 Chinese.
-
Large spectrum of HLA-C recognition by killer Ig-like receptor (KIR)2DL2 and KIR2DL3 and restricted C1 SPECIFICITY of KIR2DS2: dominant impact of KIR2DL2/KIR2DS2 on KIR2D NK cell repertoire formation.J Immunol. 2013 Nov 1;191(9):4778-88. doi: 10.4049/jimmunol.1301580. Epub 2013 Sep 27. J Immunol. 2013. PMID: 24078689
-
Killer Ig-like receptor ligand mismatch directs NK cell expansion in vitro.J Immunol. 2009 Oct 1;183(7):4502-8. doi: 10.4049/jimmunol.0803323. Epub 2009 Sep 11. J Immunol. 2009. PMID: 19748981
-
Activation of natural killer cells: underlying molecular mechanisms revealed.Scand J Immunol. 2004 Jul-Aug;60(1-2):14-22. doi: 10.1111/j.0300-9475.2004.01475.x. Scand J Immunol. 2004. PMID: 15238069 Review.
-
Structure and function of natural killer cell surface receptors.Annu Rev Biophys Biomol Struct. 2003;32:93-114. doi: 10.1146/annurev.biophys.32.110601.142347. Epub 2002 Dec 2. Annu Rev Biophys Biomol Struct. 2003. PMID: 12471063 Review.
Cited by
-
Feeder-Cell-Free and Serum-Free Expansion of Natural Killer Cells Using Cloudz Microspheres, G-Rex6M, and Human Platelet Lysate.Front Immunol. 2022 Mar 7;13:803380. doi: 10.3389/fimmu.2022.803380. eCollection 2022. Front Immunol. 2022. PMID: 35320938 Free PMC article.
-
Advances in NK cell therapy for brain tumors.NPJ Precis Oncol. 2023 Feb 15;7(1):17. doi: 10.1038/s41698-023-00356-1. NPJ Precis Oncol. 2023. PMID: 36792722 Free PMC article. Review.
-
The cell-based approach in neurosurgery: ongoing trends and future perspectives.Heliyon. 2019 Nov 26;5(11):e02818. doi: 10.1016/j.heliyon.2019.e02818. eCollection 2019 Nov. Heliyon. 2019. PMID: 31844735 Free PMC article. Review.
-
Association of Killer Cell Immunoglobulin- Like Receptor Genes in Iranian Patients with Rheumatoid Arthritis.PLoS One. 2015 Dec 11;10(12):e0143757. doi: 10.1371/journal.pone.0143757. eCollection 2015. PLoS One. 2015. PMID: 26658904 Free PMC article.
-
SOX2 immunity and tissue resident memory in children and young adults with glioma.J Neurooncol. 2017 Aug;134(1):41-53. doi: 10.1007/s11060-017-2515-8. Epub 2017 Jun 15. J Neurooncol. 2017. PMID: 28620836 Free PMC article.
References
-
- Stupp R., Mason W. P., van den Bent M. J., Weller M., Fisher B., Taphoorn M. J., Belanger K., Brandes A. A., Marosi C., Bogdahn U., et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups.; National Cancer Institute of Canada Clinical Trials Group 2005. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352: 987–996. - PubMed
-
- Ishikawa E., Takano S., Ohno T., Tsuboi K. 2012. Adoptive cell transfer therapy for malignant gliomas. Adv. Exp. Med. Biol. 746: 109–120. - PubMed
-
- Ghiringhelli F., Ménard C., Martin F., Zitvogel L. 2006. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol. Rev. 214: 229–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

