Connectivity of pacemaker neurons in the neonatal rat superficial dorsal horn
- PMID: 25380417
- PMCID: PMC4359084
- DOI: 10.1002/cne.23706
Connectivity of pacemaker neurons in the neonatal rat superficial dorsal horn
Abstract
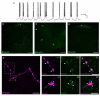
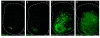
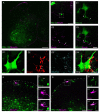
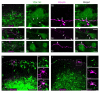
Pacemaker neurons with an intrinsic ability to generate rhythmic burst-firing have been characterized in lamina I of the neonatal spinal cord, where they are innervated by high-threshold sensory afferents. However, little is known about the output of these pacemakers, as the neuronal populations that are targeted by pacemaker axons have yet to be identified. The present study combines patch-clamp recordings in the intact neonatal rat spinal cord with tract-tracing to demonstrate that lamina I pacemaker neurons contact multiple spinal motor pathways during early life. Retrograde labeling of premotor interneurons with the trans-synaptic pseudorabies virus PRV-152 revealed the presence of burst-firing in PRV-infected lamina I neurons, thereby confirming that pacemakers are synaptically coupled to motor networks in the spinal ventral horn. Notably, two classes of pacemakers could be distinguished in lamina I based on cell size and the pattern of their axonal projections. Whereas small pacemaker neurons possessed ramified axons that contacted ipsilateral motor circuits, large pacemaker neurons had unbranched axons that crossed the midline and ascended rostrally in the contralateral white matter. Recordings from identified spino-parabrachial and spino-periaqueductal gray neurons indicated the presence of pacemaker activity within neonatal lamina I projection neurons. Overall, these results show that lamina I pacemakers are positioned to regulate both the level of activity in developing motor circuits and the ascending flow of nociceptive information to the brain, thus highlighting a potential role for pacemaker activity in the maturation of pain and sensorimotor networks in the central nervous system.
Keywords: AB_1587626; AB_2301751; AB_2336883; burst-firing; lamina I; premotor interneuron; spinal cord; synapse.
© 2015 Wiley Periodicals, Inc.
Figures


Similar articles
-
Intrinsic burst-firing in lamina I spinoparabrachial neurons during adolescence.Neurosci Lett. 2021 Apr 17;750:135794. doi: 10.1016/j.neulet.2021.135794. Epub 2021 Mar 2. Neurosci Lett. 2021. PMID: 33667599 Free PMC article.
-
Inward-rectifying K+ (Kir2) leak conductance dampens the excitability of lamina I projection neurons in the neonatal rat.Neuroscience. 2016 Dec 17;339:502-510. doi: 10.1016/j.neuroscience.2016.10.027. Epub 2016 Oct 14. Neuroscience. 2016. PMID: 27751963 Free PMC article.
-
Pacemaker neurons within newborn spinal pain circuits.J Neurosci. 2011 Jun 15;31(24):9010-22. doi: 10.1523/JNEUROSCI.6555-10.2011. J Neurosci. 2011. PMID: 21677184 Free PMC article.
-
Pacemaker Neurons and the Development of Nociception.Neuroscientist. 2014 Jun;20(3):197-202. doi: 10.1177/1073858414521499. Epub 2014 Feb 7. Neuroscientist. 2014. PMID: 24510073 Free PMC article. Review.
-
Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn.Mol Pain. 2017 Jan;13:1744806917693003. doi: 10.1177/1744806917693003. Mol Pain. 2017. PMID: 28326935 Free PMC article. Review.
Cited by
-
Stochastic slowly adapting ionic currents may provide a decorrelation mechanism for neural oscillators by causing wander in the intrinsic period.J Neurophysiol. 2016 Sep 1;116(3):1189-98. doi: 10.1152/jn.00193.2016. Epub 2016 Jun 8. J Neurophysiol. 2016. PMID: 27281746 Free PMC article.
-
Analysis of spontaneous activity of superficial dorsal horn neurons in vitro: neuropathy-induced changes.Pflugers Arch. 2016 Nov;468(11-12):2017-2030. doi: 10.1007/s00424-016-1886-6. Epub 2016 Oct 10. Pflugers Arch. 2016. PMID: 27726011
-
Presynaptic Inhibition of Primary Nociceptive Signals to Dorsal Horn Lamina I Neurons by Dopamine.J Neurosci. 2018 Oct 10;38(41):8809-8821. doi: 10.1523/JNEUROSCI.0323-18.2018. Epub 2018 Aug 24. J Neurosci. 2018. PMID: 30143577 Free PMC article.
-
Anthrax toxins regulate pain signaling and can deliver molecular cargoes into ANTXR2+ DRG sensory neurons.Nat Neurosci. 2022 Feb;25(2):168-179. doi: 10.1038/s41593-021-00973-8. Epub 2021 Dec 20. Nat Neurosci. 2022. PMID: 34931070 Free PMC article.
-
Noise or signal? Spontaneous activity of dorsal horn neurons: patterns and function in health and disease.Pflugers Arch. 2024 Aug;476(8):1171-1186. doi: 10.1007/s00424-024-02971-8. Epub 2024 Jun 1. Pflugers Arch. 2024. PMID: 38822875 Free PMC article. Review.
References
-
- Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–58. - PubMed
-
- Blumberg MS, Lucas DE. Dual mechanisms of twitching during sleep in neonatal rats. Behav Neurosci. 1994;108:1196–202. - PubMed
-
- Busetto G, Buffelli M, Cangiano L, Cangiano A. Effects of evoked and spontaneous motoneuronal firing on synapse competition and elimination in skeletal muscle. J Neurocytol. 2003;32:795–802. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

