Role of retinoic acid and platelet-derived growth factor receptor cross talk in the regulation of neonatal gonocyte and embryonal carcinoma cell differentiation
- PMID: 25380237
- PMCID: PMC5393322
- DOI: 10.1210/en.2014-1524
Role of retinoic acid and platelet-derived growth factor receptor cross talk in the regulation of neonatal gonocyte and embryonal carcinoma cell differentiation
Abstract
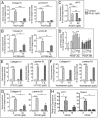
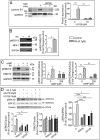
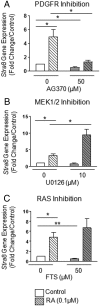
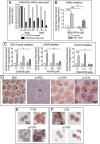
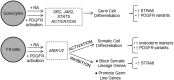
Neonatal gonocytes are direct precursors of spermatogonial stem cells, the cell pool that supports spermatogenesis. Although unipotent in vivo, gonocytes express pluripotency genes common with embryonic stem cells. Previously, we found that all-trans retinoic acid (RA) induced the expression of differentiation markers and a truncated form of platelet-derived growth factor receptor (PDGFR)β in rat gonocytes, as well as in F9 mouse embryonal carcinoma cells, an embryonic stem cell-surrogate that expresses somatic lineage markers in response to RA. The present study is focused on identifying the signaling pathways involved in RA-induced gonocyte and F9 cell differentiation. Mitogen-activated protein kinase kinase (MEK) 1/2 activation was required during F9 cell differentiation towards somatic lineage, whereas its inhibition potentiated RA-induced Stra8 expression, suggesting that MEK1/2 acts as a lineage specification switch in F9 cells. In both cell types, RA increased the expression of the spermatogonial/premeiotic marker Stra8, which is in line with F9 cells being at a stage before somatic-germline lineage specification. Inhibiting PDGFR kinase activity reduced RA-induced Stra8 expression. Interestingly, RA increased the expression of PDGFRα variant forms in both cell types. Together, these results suggest a potential cross talk between RA and PDGFR signaling pathways in cell differentiation. RA receptor-α inhibition partially reduced RA effects on Stra8 in gonocytes, indicating that RA acts in part via RA receptor-α. RA-induced gonocyte differentiation was significantly reduced by inhibiting SRC (v-src avian sarcoma [Schmidt-Ruppin A-2] viral oncogene) and JAK2/STAT5 (Janus kinase 2/signal transducer and activator of transcription 5) activities, implying that these signaling molecules play a role in gonocyte differentiation. These results suggest that gonocyte and F9 cell differentiation is regulated via cross talk between RA and PDGFRs using different downstream pathways.
Figures



Similar articles
-
Identification and distribution of a novel platelet-derived growth factor receptor beta variant: effect of retinoic acid and involvement in cell differentiation.Endocrinology. 2007 May;148(5):2233-50. doi: 10.1210/en.2006-1206. Epub 2007 Feb 15. Endocrinology. 2007. PMID: 17303670
-
Platelet-derived growth factor receptor beta-subtype regulates proliferation and migration of gonocytes.Endocrinology. 2008 Dec;149(12):6226-35. doi: 10.1210/en.2008-0349. Epub 2008 Aug 7. Endocrinology. 2008. PMID: 18687785
-
Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro.Biol Reprod. 2008 Mar;78(3):537-45. doi: 10.1095/biolreprod.107.064337. Epub 2007 Nov 21. Biol Reprod. 2008. PMID: 18032419 Free PMC article.
-
Retinoic Acid Signaling in P19 Stem Cell Differentiation.Anticancer Agents Med Chem. 2017;17(9):1184-1198. doi: 10.2174/1871520616666160615065000. Anticancer Agents Med Chem. 2017. PMID: 27306567 Review.
-
How does retinoic acid (RA) signaling pathway regulate spermatogenesis?Histol Histopathol. 2022 Nov;37(11):1053-1064. doi: 10.14670/HH-18-478. Epub 2022 Jun 6. Histol Histopathol. 2022. PMID: 35673893 Review.
Cited by
-
GLIS1-3: emerging roles in reprogramming, stem and progenitor cell differentiation and maintenance.Stem Cell Investig. 2017 Sep 27;4:80. doi: 10.21037/sci.2017.09.01. eCollection 2017. Stem Cell Investig. 2017. PMID: 29057252 Free PMC article. Review.
-
Retinoic Acid Induces Differentiation of Mouse F9 Embryonic Carcinoma Cell by Modulating the miR-485 Targeting of Abhd2.Int J Mol Sci. 2019 Apr 26;20(9):2071. doi: 10.3390/ijms20092071. Int J Mol Sci. 2019. PMID: 31035455 Free PMC article.
-
Protective Role of Peroxiredoxins against Reactive Oxygen Species in Neonatal Rat Testicular Gonocytes.Antioxidants (Basel). 2019 Dec 30;9(1):32. doi: 10.3390/antiox9010032. Antioxidants (Basel). 2019. PMID: 31905831 Free PMC article.
-
Regulation of Translocator Protein 18 kDa (TSPO) Expression in Rat and Human Male Germ Cells.Int J Mol Sci. 2016 Sep 6;17(9):1486. doi: 10.3390/ijms17091486. Int J Mol Sci. 2016. PMID: 27608010 Free PMC article.
-
Hypermaintenance and hypofunction of aged spermatogonia: insight from age-related increase of Plzf expression.Oncotarget. 2015 Jun 30;6(18):15891-901. doi: 10.18632/oncotarget.4045. Oncotarget. 2015. PMID: 25986924 Free PMC article.
References
-
- Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today. 2009;876:1–26. - PubMed
-
- Culty M. Gonocytes, from the fifties to the present: is there a reason to change the name? Biol Reprod. 2013;89(2):46. - PubMed
-
- Orth JM, Boehm R. Functional coupling of neonatal rat Sertoli cells and gonocytes in coculture. Endocrinology. 1990;127:2812–2820. - PubMed
-
- McGuinness MP, Orth JM. Reinitiation of gonocyte mitosis and movement of gonocytes to the basement membrane in testes of newborn rats in vivo and in vitro. Anat Rec. 1992;233:527–537. - PubMed
-
- McGuinness MP, Orth JM. Gonocytes of male rats resume migratory activity postnatally. Eur J Cell Biol. 1992;59:196–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

