Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling
- PMID: 25375408
- PMCID: PMC4365061
- DOI: 10.2119/molmed.2014.00076
Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling
Abstract
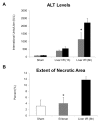
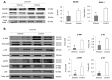
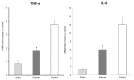
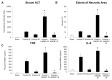
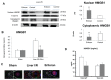
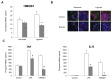
Toll-like receptor 4 (TLR4) is ubiquitously expressed on parenchymal and immune cells of the liver and is the most studied TLR responsible for the activation of proinflammatory signaling cascades in liver ischemia and reperfusion (I/R). Since pharmacological inhibition of TLR4 during the sterile inflammatory response of I/R has not been studied, we sought to determine whether eritoran, a TLR4 antagonist trialed in sepsis, could block hepatic TLR4-mediated inflammation and end organ damage. When C57BL/6 mice were pretreated with eritoran and subjected to warm liver I/R, there was significantly less hepatocellular injury compared to control counterparts. Additionally, we found that eritoran is protective in liver I/R through inhibition of high-mobility group box protein B1 (HMGB1)-mediated inflammatory signaling. When eritoran was administered in conjunction with recombinant HMGB1 during liver I/R, there was significantly less injury, suggesting that eritoran blocks the HMGB1-TLR4 interaction. Not only does eritoran attenuate TLR4-dependent HMGB1 release in vivo, but this TLR4 antagonist also dampened HMGB1's release from hypoxic hepatocytes in vitro and thereby weakened HMGB1's activation of innate immune cells. HMGB1 signaling through TLR4 makes an important contribution to the inflammatory response seen after liver I/R. This study demonstrates that novel blockade of HMGB1 by the TLR4 antagonist eritoran leads to the amelioration of liver injury.
Figures
Similar articles
-
Novel strategies for targeting innate immune responses to influenza.Mucosal Immunol. 2016 Sep;9(5):1173-82. doi: 10.1038/mi.2015.141. Epub 2016 Jan 27. Mucosal Immunol. 2016. PMID: 26813341 Free PMC article.
-
Paeoniflorin protects against liver ischemia/reperfusion injury in mice via inhibiting HMGB1-TLR4 signaling pathway.Phytother Res. 2018 Nov;32(11):2247-2255. doi: 10.1002/ptr.6161. Epub 2018 Jul 26. Phytother Res. 2018. PMID: 30047580
-
Dexmedetomidine Post-Conditioning Alleviates Cerebral Ischemia-Reperfusion Injury in Rats by Inhibiting High Mobility Group Protein B1 Group (HMGB1)/Toll-Like Receptor 4 (TLR4)/Nuclear Factor kappa B (NF-κB) Signaling Pathway.Med Sci Monit. 2020 Jan 8;26:e918617. doi: 10.12659/MSM.918617. Med Sci Monit. 2020. PMID: 31912804 Free PMC article.
-
Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia.Front Biosci (Schol Ed). 2010 Jun 1;2(3):1081-91. doi: 10.2741/s119. Front Biosci (Schol Ed). 2010. PMID: 20515842 Review.
-
Eritoran tetrasodium (E5564) treatment for sepsis: review of preclinical and clinical studies.Expert Opin Drug Metab Toxicol. 2011 Apr;7(4):479-94. doi: 10.1517/17425255.2011.558190. Epub 2011 Feb 17. Expert Opin Drug Metab Toxicol. 2011. PMID: 21323610 Free PMC article. Review.
Cited by
-
Metformin Attenuates Ischemia-reperfusion Injury of Fatty Liver in Rats Through Inhibition of the TLR4/NF-κB Axis.Balkan Med J. 2020 Jun 1;37(4):196-202. doi: 10.4274/balkanmedj.galenos.2020.2019.9.31. Epub 2020 Apr 9. Balkan Med J. 2020. PMID: 32270948 Free PMC article.
-
Mitochondrial Dysfunction and Autophagy in Hepatic Ischemia/Reperfusion Injury.Biomed Res Int. 2015;2015:183469. doi: 10.1155/2015/183469. Epub 2015 Dec 6. Biomed Res Int. 2015. PMID: 26770970 Free PMC article. Review.
-
Combined bacterial translocation and cholestasis aggravates liver injury by activation pyroptosis in obstructive jaundice.Heliyon. 2024 Aug 3;10(16):e35793. doi: 10.1016/j.heliyon.2024.e35793. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220957 Free PMC article.
-
Responses of hepatic sinusoidal cells to liver ischemia-reperfusion injury.Front Cell Dev Biol. 2023 Apr 4;11:1171317. doi: 10.3389/fcell.2023.1171317. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37082623 Free PMC article. Review.
-
Innate immune modulation in transplantation: mechanisms, challenges, and opportunities.Front Transplant. 2023 Dec 8;2:1277669. doi: 10.3389/frtra.2023.1277669. eCollection 2023. Front Transplant. 2023. PMID: 38993914 Free PMC article. Review.
References
-
- Tsung A, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in non-parenchymal cells. J Immunol. 2005;175:7661–8. - PubMed
-
- Abu-Amara M, et al. Liver ischemia/reperfusion injury: processes in inflammatory networks: a review. Liver Transpl. 2010;16:1016–32. - PubMed
-
- Wu HS, et al. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2004;3:250–3. - PubMed
-
- Zhai Y, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

