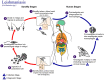
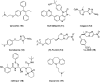
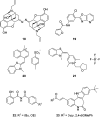
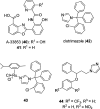
Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis
- PMID: 25365529
- PMCID: PMC4633805
- DOI: 10.1021/cr500365f
Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis
Figures


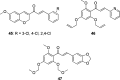
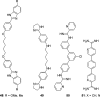
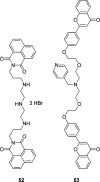
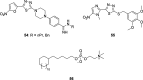



Similar articles
-
[Chemotherapy of leishmaniasis and human trypanosomiasis: status and new developments].Pharm Unserer Zeit. 1999 Jul;28(4):177-85. Pharm Unserer Zeit. 1999. PMID: 10443145 Review. German. No abstract available.
-
[Chemotherapeutic effectiveness of stibomen in experimental leishmaniasis and trypanosomiasis].Med Parazitol (Mosk). 1967 Jul-Aug;36(4):422-7. Med Parazitol (Mosk). 1967. PMID: 5612807 Russian. No abstract available.
-
Trypanosomiais and leishmaniasis "recent development in the chemotherapy of infectious diseases caused by parasitic protozoa".Curr Pharm Des. 2008;14(9):821. doi: 10.2174/138161208784041097. Curr Pharm Des. 2008. PMID: 18473831 No abstract available.
-
A review of QSAR studies to discover new drug-like compounds actives against leishmaniasis and trypanosomiasis.Curr Top Med Chem. 2012;12(8):852-65. doi: 10.2174/156802612800166756. Curr Top Med Chem. 2012. PMID: 22352913 Review.
-
Computer-Aided Drug Discovery Approaches against the Tropical Infectious Diseases Malaria, Tuberculosis, Trypanosomiasis, and Leishmaniasis.ACS Infect Dis. 2016 Jan 8;2(1):8-31. doi: 10.1021/acsinfecdis.5b00093. Epub 2015 Nov 16. ACS Infect Dis. 2016. PMID: 27622945
Cited by
-
Exploring 7β-amino-6-nitrocholestens as COVID-19 antivirals: in silico, synthesis, evaluation, and integration of artificial intelligence (AI) in drug design: assessing the cytotoxicity and antioxidant activity of 3β-acetoxynitrocholestane.RSC Med Chem. 2024 Sep 26. doi: 10.1039/d4md00257a. Online ahead of print. RSC Med Chem. 2024. PMID: 39430952 Free PMC article.
-
Medicinal plants with promising antileishmanial activity in Ethiopia: A systematic review and meta-analysis.Medicine (Baltimore). 2024 May 31;103(22):e38480. doi: 10.1097/MD.0000000000038480. Medicine (Baltimore). 2024. PMID: 39259058 Free PMC article.
-
Identification of Novel Antileishmanial Chemotypes By High-Throughput Virtual and In Vitro Screening.Acta Parasitol. 2024 Sep;69(3):1439-1457. doi: 10.1007/s11686-024-00899-8. Epub 2024 Aug 16. Acta Parasitol. 2024. PMID: 39150581
-
Aminopyridines in the development of drug candidates against protozoan neglected tropical diseases.Future Med Chem. 2024 Jul 2;16(13):1357-1373. doi: 10.1080/17568919.2024.2359361. Epub 2024 Jun 10. Future Med Chem. 2024. PMID: 39109436 Review.
-
An integrated bioinformatic analysis of microarray datasets to identify biomarkers and miRNA-based regulatory networks in leishmaniasis.Sci Rep. 2024 Jun 5;14(1):12981. doi: 10.1038/s41598-024-63462-5. Sci Rep. 2024. PMID: 38839916 Free PMC article.
References
-
- DNDi Home Page. http://www.dndi.org/diseases-projects/diseases/vl/tpp/tpp-vl.html. (accessed 08/31/2014).
-
- Manson-Bahr P. E. C.The Wellcome Trust illustrated history of tropical diseases; Cox F. E. G., Ed., The Wellcome Trust: London, U.K., 1996.
-
- Ross M. R.Report on the nature of kala azar; Office of the superintendent of government printing: Calcutta, India, 1899; pp 1–104.
-
- Hoare C. A. Trans. R. Soc. Trop. Med. Hyg. 1938, 32, 66.
-
- Nicolle C. C. R. Hebd. Seances Acad. Sci. 1908, 146, 842.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

