Impact and regulation of lambda interferon response in human metapneumovirus infection
- PMID: 25355870
- PMCID: PMC4301146
- DOI: 10.1128/JVI.02897-14
Impact and regulation of lambda interferon response in human metapneumovirus infection
Abstract
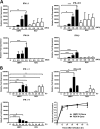
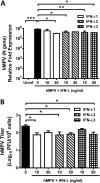
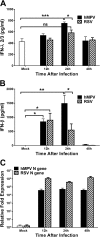
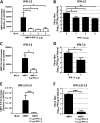
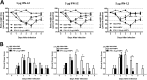
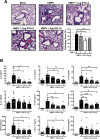
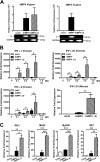
Human metapneumovirus (hMPV) is a respiratory paramyxovirus that is distributed worldwide and induces significant airway morbidity. Despite the relevance of hMPV as a pathogen, many aspects of the immune response to this virus are still largely unknown. In this report, we focus on the antiviral immune response, which is critical for viral clearance and disease resolution. Using in vitro and in vivo systems, we show that hMPV is able to induce expression of lambda interferon 1 (IFN-λ1), IFN-λ2, IFN-λ3, and IFN-λ4. The induction of IFN-λ expression by hMPV was dependent on interferon regulatory factor 7 (IRF-7) expression but not on IRF-3 expression. Treatment of hMPV-infected mice with IFN-λ reduced the disease severity, lung viral titer, and inflammatory response in the lung. Moreover, the IFN-λ response induced by the virus was regulated by the expression of the hMPV G protein. These results show that type III interferons (IFN-λs) play a critical protective role in hMPV infection.
Importance: Human metapneumovirus (hMPV) is a pathogen of worldwide importance. Despite the relevance of hMPV as a pathogen, critical aspects of the immune response induced by this virus remain unidentified. Interferons (IFNs), including IFN-λ, the newest addition to the interferon family, constitute an indispensable part of the innate immune response. Here, we demonstrated that IFN-λ exhibited a protective role in hMPV infection in vitro and in an experimental mouse model of infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
IFN-λ drives distinct lung immune landscape changes and antiviral responses in human metapneumovirus infection.mBio. 2024 May 8;15(5):e0055024. doi: 10.1128/mbio.00550-24. Epub 2024 Mar 26. mBio. 2024. PMID: 38530032 Free PMC article.
-
Critical role of MDA5 in the interferon response induced by human metapneumovirus infection in dendritic cells and in vivo.J Virol. 2013 Jan;87(2):1242-51. doi: 10.1128/JVI.01213-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152520 Free PMC article.
-
Role of type I interferon signaling in human metapneumovirus pathogenesis and control of viral replication.J Virol. 2015 Apr;89(8):4405-20. doi: 10.1128/JVI.03275-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653440 Free PMC article.
-
Interferon-Mediated Response to Human Metapneumovirus Infection.Viruses. 2018 Sep 18;10(9):505. doi: 10.3390/v10090505. Viruses. 2018. PMID: 30231515 Free PMC article. Review.
-
Human Metapneumovirus: Mechanisms and Molecular Targets Used by the Virus to Avoid the Immune System.Front Immunol. 2018 Oct 24;9:2466. doi: 10.3389/fimmu.2018.02466. eCollection 2018. Front Immunol. 2018. PMID: 30405642 Free PMC article. Review.
Cited by
-
Interferon Epsilon-Mediated Antiviral Activity Against Human Metapneumovirus and Respiratory Syncytial Virus.Vaccines (Basel). 2024 Oct 21;12(10):1198. doi: 10.3390/vaccines12101198. Vaccines (Basel). 2024. PMID: 39460364 Free PMC article.
-
Human Metapneumovirus-Induced Host microRNA Expression Impairs the Interferon Response in Macrophages and Epithelial Cells.Viruses. 2023 Nov 18;15(11):2272. doi: 10.3390/v15112272. Viruses. 2023. PMID: 38005948 Free PMC article.
-
Dengue virus infection induces interferon-lambda1 to facilitate cell migration.Sci Rep. 2016 Jul 26;6:24530. doi: 10.1038/srep24530. Sci Rep. 2016. PMID: 27456172 Free PMC article.
-
Macrophage Coordination of the Interferon Lambda Immune Response.Front Immunol. 2019 Nov 19;10:2674. doi: 10.3389/fimmu.2019.02674. eCollection 2019. Front Immunol. 2019. PMID: 31798594 Free PMC article.
-
Clean and folded: Production of active, high quality recombinant human interferon-λ1.Process Biochem. 2021 Dec;111:32-39. doi: 10.1016/j.procbio.2021.08.029. Epub 2021 Sep 2. Process Biochem. 2021. PMID: 34493923 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

