Aggregation properties of the small nuclear ribonucleoprotein U1-70K in Alzheimer disease
- PMID: 25355317
- PMCID: PMC4271217
- DOI: 10.1074/jbc.M114.562959
Aggregation properties of the small nuclear ribonucleoprotein U1-70K in Alzheimer disease
Abstract
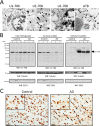
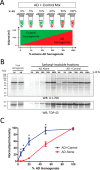
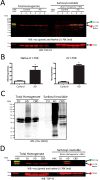
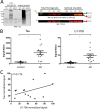
Recent evidence indicates that U1-70K and other U1 small nuclear ribonucleoproteins are Sarkosyl-insoluble and associate with Tau neurofibrillary tangles selectively in Alzheimer disease (AD). Currently, the mechanisms underlying the conversion of soluble nuclear U1 small nuclear ribonucleoproteins into insoluble cytoplasmic aggregates remain elusive. Based on the biochemical and subcellular distribution properties of U1-70K in AD, we hypothesized that aggregated U1-70K itself or other biopolymers (e.g. proteins or nucleic acids) interact with and sequester natively folded soluble U1-70K into insoluble aggregates. Here, we demonstrate that total homogenates from AD brain induce soluble U1-70K from control brain or recombinant U1-70K to become Sarkosyl-insoluble. This effect was not dependent on RNA and did not correlate with detergent-insoluble Tau levels as AD homogenates with reduced levels of these components were still capable of inducing U1-70K aggregation. In contrast, proteinase K-treated AD homogenates and Sarkosyl-soluble AD fractions were unable to induce U1-70K aggregation, indicating that aggregated proteins in AD brain are responsible for inducing soluble U1-70K aggregation. It was determined that the C terminus of U1-70K, which harbors two disordered low complexity (LC) domains, is necessary for U1-70K aggregation. Moreover, both LC1 and LC2 domains were sufficient for aggregation. Finally, protein cross-linking and mass spectrometry studies demonstrated that a U1-70K fragment harboring the LC1 domain directly interacts with aggregated U1-70K in AD brain. Our results support a hypothesis that aberrant forms of U1-70K in AD can directly sequester soluble forms of U1-70K into insoluble aggregates.
Keywords: Neurodegenerative Disease; Protein Aggregation; RNA; Spliceosome; Tau Protein (Tau).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
RNA-binding proteins with basic-acidic dipeptide (BAD) domains self-assemble and aggregate in Alzheimer's disease.J Biol Chem. 2018 Jul 13;293(28):11047-11066. doi: 10.1074/jbc.RA118.001747. Epub 2018 May 25. J Biol Chem. 2018. PMID: 29802200 Free PMC article.
-
Changes in the detergent-insoluble brain proteome linked to amyloid and tau in Alzheimer's Disease progression.Proteomics. 2016 Dec;16(23):3042-3053. doi: 10.1002/pmic.201600057. Proteomics. 2016. PMID: 27718298 Free PMC article.
-
Enrichment of Detergent-insoluble Protein Aggregates from Human Postmortem Brain.J Vis Exp. 2017 Oct 24;(128):55835. doi: 10.3791/55835. J Vis Exp. 2017. PMID: 29155708 Free PMC article.
-
Deep Profiling of the Aggregated Proteome in Alzheimer's Disease: From Pathology to Disease Mechanisms.Proteomes. 2018 Nov 12;6(4):46. doi: 10.3390/proteomes6040046. Proteomes. 2018. PMID: 30424485 Free PMC article. Review.
-
Mass spectrometric insights into protein aggregation.Essays Biochem. 2023 Mar 29;67(2):243-253. doi: 10.1042/EBC20220103. Essays Biochem. 2023. PMID: 36636963 Free PMC article. Review.
Cited by
-
Rho Kinase Inhibition as a Therapeutic for Progressive Supranuclear Palsy and Corticobasal Degeneration.J Neurosci. 2016 Jan 27;36(4):1316-23. doi: 10.1523/JNEUROSCI.2336-15.2016. J Neurosci. 2016. PMID: 26818518 Free PMC article.
-
Evidence of Filamin A loss of solubility at the prodromal stage of neuropathologically-defined Alzheimer's disease.Front Aging Neurosci. 2022 Nov 24;14:1038343. doi: 10.3389/fnagi.2022.1038343. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36506473 Free PMC article.
-
Oligomeric, phosphorylated, and truncated tau and spliceosome pathology within the entorhinal-hippocampal connectome across stages of Alzheimer's disease.J Comp Neurol. 2023 Dec;531(18):2080-2108. doi: 10.1002/cne.25466. Epub 2023 Mar 29. J Comp Neurol. 2023. PMID: 36989381 Free PMC article.
-
A Multi-network Approach Identifies Protein-Specific Co-expression in Asymptomatic and Symptomatic Alzheimer's Disease.Cell Syst. 2017 Jan 25;4(1):60-72.e4. doi: 10.1016/j.cels.2016.11.006. Epub 2016 Dec 15. Cell Syst. 2017. PMID: 27989508 Free PMC article.
-
Heparin-enriched plasma proteome is significantly altered in Alzheimer's disease.Mol Neurodegener. 2024 Oct 8;19(1):67. doi: 10.1186/s13024-024-00757-1. Mol Neurodegener. 2024. PMID: 39380021 Free PMC article.
References
-
- Taylor J. P., Hardy J., Fischbeck K. H. (2002) Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 - PubMed
-
- Ross C. A., Poirier M. A. (2004) Protein aggregation and neurodegenerative disease. Nat. Med. 10, S10–S17 - PubMed
-
- Querfurth H. W., LaFerla F. M. (2010) Alzheimer's disease. New Engl. J. Med. 362, 329–344 - PubMed
-
- Sperling R. A., Aisen P. S., Beckett L. A., Bennett D. A., Craft S., Fagan A. M., Iwatsubo T., Jack C. R., Jr., Kaye J., Montine T. J., Park D. C., Reiman E. M., Rowe C. C., Siemers E., Stern Y., Yaffe K., Carrillo M. C., Thies B., Morrison-Bogorad M., Wagster M. V., Phelps C. H. (2011) Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia 7, 280–292 - PMC - PubMed
-
- O'Brien R. J., Resnick S. M., Zonderman A. B., Ferrucci L., Crain B. J., Pletnikova O., Rudow G., Iacono D., Riudavets M. A., Driscoll I., Price D. L., Martin L. J., Troncoso J. C. (2009) Neuropathologic Studies of the Baltimore Longitudinal Study of Aging (BLSA). J. Alzheimer's Dis. 18, 665–675 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

