Tolerability of selective cyclooxygenase 2 inhibitors used for the treatment of rheumatological manifestations of inflammatory bowel disease
- PMID: 25340915
- PMCID: PMC11200115
- DOI: 10.1002/14651858.CD007744.pub2
Tolerability of selective cyclooxygenase 2 inhibitors used for the treatment of rheumatological manifestations of inflammatory bowel disease
Abstract
Background: Nonsteroidal anti-inflammatory drugs (NSAIDs) are used to reduce inflammatory pain and swelling in inflammatory bowel disease (IBD) patients with rheumatological manifestations. While these drugs effectively reduce musculoskeletal pain and stiffness, long-term use is limited by gastrointestinal (GI) adverse effects (AEs) and disease exacerbation. As an alternative to NSAIDs, selective cyclooxygenase 2 (COX-2) inhibitors were developed to improve GI safety and tolerability. COX-2 inhibitors include drugs such as celecoxib, rofecoxib, valdecoxib, etoricoxib, and lumiracoxib. Rofecoxib and valdecoxib have been withdrawn from the market worldwide due to safety concerns (most importantly for cardiovascular adverse events) and lumiracoxib has been withdrawn in many countries due to liver toxicity. However, celecoxib and etoricoxib continue to be available for use in many countries. Several studies have examined whether COX-2 inhibitors can be safely used for the treatment of rheumatological manifestations of IBD with inconsistent results. Some investigators report acceptable safety profiles associated with these drugs while others found that COX-2 inhibitors are associated with high rates of disease exacerbation.
Objectives: The objective of this systematic review was to evaluate the tolerability and safety of COX-2 inhibitors used for the treatment of rheumatological manifestations of IBD.
Search methods: We searched the following databases from inception to 19 September 2013: PubMed, EMBASE, MEDLINE and CENTRAL. The search was not limited by language. Additional trials were identified by manually searching the reference lists of relevant papers and conference proceedings and through correspondence with experts and pharmaceutical companies.
Selection criteria: Randomized controlled trials (RCTs) that compared COX-2 inhibitors to placebo were considered for inclusion. Participants were adult patients with IBD presenting with rheumatological manifestations of at least two weeks duration.
Data collection and analysis: Two authors independently assessed trial eligibility and extracted data. Methodological quality was assessed using the Cochrane risk of bias tool. The primary outcome measure was the proportion of patients with disease exacerbation as defined by the included studies. Secondary outcomes included GI adverse effects, renal toxicity, cardiovascular and thrombotic events. Data were analysed on an intention-to-treat basis where patients with missing final outcomes were assumed to have had an exacerbation of IBD. We calculated the risk ratio (RR) and corresponding 95% confidence interval (95% CI) for dichotomous outcomes. The overall quality of the evidence was assessed using the GRADE criteria.
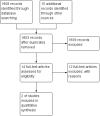
Main results: There were no RCTs that assessed the tolerability or safety of the withdrawn COX-2 inhibitors rofecoxib, valdecoxib, or lumiracoxib. Two RCTs (n = 381 IBD patients with rheumatological manifestations) were included in the review. One study (n = 159) compared etoricoxib (60 to 120 mg/day) to placebo in IBD patients with quiescent or active ulcerative colitis or Crohn's disease. The other study (n = 222) compared celecoxib (200 mg twice daily) to placebo in patients with quiescent ulcerative colitis. Both studies were judged to be at low risk of bias. The two included studies were not pooled for meta-analysis due to differences in patient populations and treatment duration. There was no statistically significant difference in exacerbation of IBD between etoricoxib and placebo. After 12 weeks of treatment the IBD exacerbation rate was 17% (14/82) in the etoricoxib group compared to 19% (15/77) in the placebo group (RR 0.88, 95% CI 0.45 to 1.69). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (29 events). There was no statistically significant difference in exacerbation of ulcerative colitis between celecoxib and placebo. After two weeks of treatment 4% (5/112) of celecoxib patients experienced an exacerbation of ulcerative colitis compared to 6% (7/110) of patients in the placebo group (RR 0.70, 95% CI 0.23 to 2.14). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (12 events). The study comparing etoricoxib to placebo documented but did not report on AEs. The proportion of patients who experienced AEs was similar in the celecoxib and placebo groups (21% and 17%, respectively, P > 0.20). No patients in either group died or experienced serious adverse events. Eleven percent of patients in the celecoxib and placebo groups experienced GI AEs (RR 0.97, 95% CI 0.46 to 2.07). A GRADE analysis indicated that the overall quality of the evidence supporting this outcome was low due to very sparse data (24 events). GI AEs led to premature withdrawal from the study in 3% of patients in celecoxib and placebo groups respectively. GI AEs included increased stool frequency, rectal bleeding, and inflamed mucosa. No patients experienced any cardiovascular adverse events. Renal toxicity or thrombotic AEs were not reported.
Authors' conclusions: The results for disease exacerbation and AEs between the COX-2 inhibitors celecoxib and etoricoxib and placebo were uncertain. Thus no definitive conclusions regarding the tolerability and safety of the short term use of celecoxib and etoricoxib in patients with IBD can be drawn. The two included studies suggest that celecoxib and etoricoxib do not exacerbate IBD symptoms. However, it should be noted that both studies had relatively small sample sizes and short follow-up durations. Clinicians need to continue to weigh the risks and benefits of these drugs when treating patients IBD patients with rheumatological manifestations in order to avoid disease exacerbation and other adverse effects. Further RCTs are needed to determine the tolerability and safety of celecoxib and etoricoxib in these patients.
Conflict of interest statement
None known.
Figures







Update of
- doi: 10.1002/14651858.CD007744
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.Health Technol Assess. 2008 Apr;12(11):1-278, iii. doi: 10.3310/hta12110. Health Technol Assess. 2008. PMID: 18405470 Review.
-
Role of selective cyclooxygenase-2 inhibitors in exacerbation of inflammatory bowel disease: A systematic review and meta-analysis.Curr Ther Res Clin Exp. 2008 Jun;69(3):181-91. doi: 10.1016/j.curtheres.2008.06.009. Curr Ther Res Clin Exp. 2008. PMID: 24692797 Free PMC article.
-
Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia.Cochrane Database Syst Rev. 2020 Apr 30;4(4):CD011459. doi: 10.1002/14651858.CD011459.pub2. Cochrane Database Syst Rev. 2020. PMID: 32352165 Free PMC article.
-
Aminosalicylates for induction of remission or response in Crohn's disease.Cochrane Database Syst Rev. 2016 Jul 3;7(7):CD008870. doi: 10.1002/14651858.CD008870.pub2. Cochrane Database Syst Rev. 2016. PMID: 27372735 Free PMC article. Review.
Cited by
-
Renal insufficiency plays a crucial association factor in severe knee osteoarthritis-induced pain in patients with total knee replacement: A retrospective study.Medicine (Baltimore). 2020 Feb;99(6):e19125. doi: 10.1097/MD.0000000000019125. Medicine (Baltimore). 2020. PMID: 32028438 Free PMC article.
-
Phytotherapies in inflammatory bowel disease.J Res Med Sci. 2019 May 22;24:42. doi: 10.4103/jrms.JRMS_590_17. eCollection 2019. J Res Med Sci. 2019. PMID: 31160909 Free PMC article. Review.
-
British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults.Gut. 2019 Dec;68(Suppl 3):s1-s106. doi: 10.1136/gutjnl-2019-318484. Epub 2019 Sep 27. Gut. 2019. PMID: 31562236 Free PMC article. Review.
-
Modulation of genomic and epigenetic end-points by celecoxib.Oncotarget. 2018 Sep 14;9(72):33656-33681. doi: 10.18632/oncotarget.26062. eCollection 2018 Sep 14. Oncotarget. 2018. PMID: 30263093 Free PMC article.
-
Macroalgal Proteins: A Review.Foods. 2022 Feb 16;11(4):571. doi: 10.3390/foods11040571. Foods. 2022. PMID: 35206049 Free PMC article. Review.
References
References to studies included in this review
El Miedany 2006 {published data only}
-
- Miedany Y, Youssef S, Ahmed I, Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox‐2 inhibitor in inflammatory bowel diseases. American Journal of Gastroenterology 2006;101(2):311‐7. - PubMed
Sandborn 2006 {published data only}
-
- Sandborn WJ, Stenson WF, Brynskov J, Lorenz RG, Steidle GM, Robbins JL, et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo‐controlled, pilot study. Clinical Gastroenterology and Hepatology 2006;4(2):203‐11. - PubMed
References to studies excluded from this review
Biancone 2004 {published data only}
-
- Biancone L, Tosti C, Geremia A, Fina D, Petruzziello C, Emerenziani S, et al. Rofecoxib and early relapse of inflammatory bowel disease: An open‐label trial. Alimentary Pharmacology and Therapeutics 2004;19(7):755‐64. - PubMed
Bonner 2001 {published data only}
-
- Bonner GF. Exacerbation of inflammatory bowel disease associated with use of celecoxib. American Journal of Gastroenterology 2001;96(4):1306‐8. - PubMed
Charachon A 2003 {published data only}
-
- Charachon A, Petit T, Lamarque D, Soulé JC. Acute ulcerative colitis in a patient treated with rofecoxib who took aspirin as self‐medication. Gastroentérologie Clinique et Biologique. 2003;27(5):511‐3. - PubMed
Freedman 2002 {published data only}
-
- Freedman GM, Kreitzer JM, Badola R. Rofecoxib‐associated upper gastrointestinal bleed: a case report. Mount Sinai Journal of Medicine 2002;69(1‐2):105‐6. - PubMed
Goh 2002 {published data only}
-
- Goh J, Wight D, Parkes M, Middleton SJ, Hunter JO. Rofecoxib and cytomegalovirus in acute flare‐up of ulcerative colitis: coprecipitants or coincidence?. American Journal of Gastroenterology 2002;97(4):1061‐2. - PubMed
Gornet 2002 {published data only}
-
- Gornet JM, Hassani Z, Modiglian R, Lémann M. Exacerbation of Crohn's colitis with severe colonic hemorrhage in a patient on rofecoxib. American Journal of Gastroenterology 2002;97(12):3209‐10. - PubMed
Mahadevant 2002 {published data only}
-
- Mahadevan U, Loftus EV Jr, Tremaine WJ, Sandborn WJ. Safety of selective cyclooxygenase‐2 inhibitors in inflammatory bowel disease. American Journal of Gastroenterology 2002;97(4):910‐4. - PubMed
Matuk 2004 {published data only}
-
- Matuk R, Crawford J, Abreu MT, Targan SR, Vasiliauskas EA, Papadakis KA. The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase‐2 inhibitors in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2004;10(4):352‐6. - PubMed
Reinisch 2003 {published data only}
-
- Reinisch W, Miehsler W, Dejaco C, Harrer M, Waldhoer T, Lichtenberger C, et al. An open‐label trial of the selective cyclo‐oxygenase‐2 inhibitor, rofecoxib, in inflammatory bowel disease‐associated peripheral arthritis and arthralgia. Alimentary Pharmacology and Therapeutics 2003;17(11):1371‐80. - PubMed
Rey 2005 {published data only}
-
- Rey P, Andriamanantena D, Carrère C, Casassus‐Builhè D, Hamant N, Perret JL. Ulcerating haemorrhagic colitis induced by celecoxib. Presse Médicale 2005;34(6):443‐5. - PubMed
Takeuchi 2006 {published data only}
-
- Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, et al. Prevalence and mechanism of nonsteroidal anti‐inflammatory drug‐induced clinical relapse in patients with inflammatory bowel disease. Clinical Gastroenterology and Hepatology 2006;4(2):196‐202. - PubMed
Wilcox 2005 {published data only}
-
- Wilcox GM, Mattia AR. Rofecoxib and inflammatory bowel disease: clinical and pathologic observations. Journal of Clinical Gastroenterology 2005;39(2):142‐3. - PubMed
References to studies awaiting assessment
Additional references
Andres 1999
-
- Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterology Clinics of North America 1999;28(2):255‐81. - PubMed
Atukorala 2013
-
- Atukorala I, Hunter DJ. Valdecoxib : the rise and fall of a COX‐2 inhibitor. Expert Opinion on Pharmacotherapy 2013;14(8):1077‐86. - PubMed
Beaugerie 2004
-
- Beaugerie L, Thiéfin G. Gastrointestinal complications related to NSAIDs. Gastroentérologie Clinique et Biologique 2004;28 Spec No 3:C62‐72. - PubMed
Bjarnason 1993
-
- Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti‐inflammatory drugs on the small and large intestine in humans. Gastroenterology 1993;104(6):1832‐47. - PubMed
Bombardier 2000
-
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos‐Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. New England Journal of Medicine 2000;343(21):1520‐8. - PubMed
Brophy 2005
-
- Brophy JM. Cardiovascular risk associated with celecoxib. New England Journal of Medicine 2005;352(25):2648‐50; author reply 2648‐50. - PubMed
Byerly 2005
-
- Byerly FL, Nelson KC, Granko RP, Morrell DS, Cairns BA. Valdecoxib‐associated acute generalized exanthematous pustulosis. Burns 2005;31(3):383‐7. - PubMed
Crofford 2000
-
- Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, Putte LB. Basic biology and clinical application of specific cyclooxygenase‐2 inhibitors. Arthritis and Rheumatism 2000;43(1):4‐13. - PubMed
De Vecchis 2014
-
- Vecchis R, Baldi C, Biase G, Ariano C, Cioppa C, Giasi A, et al. Cardiovascular risk associated with celecoxib or etoricoxib: a meta‐analysis of randomized controlled trials which adopted comparison with placebo or naproxen. Minerva Cardioangiologica 2014 Jul 16. [Epub ahead of print]. - PubMed
Egger 1997
Finch 1989
-
- Finch W. Arthritis and the gut. Postgraduate Medicine 1989;86(2):229‐30, 233‐4. - PubMed
Fok 2013
-
- Fok KC, Bell CJ, Read RB, Eckstein RP, Jones BE. Lumiracoxib‐induced cholestatic liver injury. Internal Medicine Journal 2013;43(6):731‐2. - PubMed
Furberg 2005
-
- Furberg CD, Psaty BM, FitzGerald GA. Parecoxib, valdecoxib, and cardiovascular risk. Circulation 2005;111(3):249. - PubMed
Gravallese 1988
-
- Gravallese EM, Kantrowitz FG. Arthritic manifestations of inflammatory bowel disease. American Journal of Gastroenterology 1988;83(7):703‐9. - PubMed
Greenstein 1976
-
- Greenstein AJ, Janowitz HD, Sachar DB. The extra‐intestinal complications of Crohn's disease and ulcerative colitis: A study of 700 patients. Medicine (Baltimore) 1976;55(5):401‐12. - PubMed
Griffin 2001
-
- Griffin MR, Scheiman JM. Prospects for changing the burden of nonsteroidal anti‐inflammatory drug toxicity. American Journal of Medicine 2001;110(1A):33S‐7S. - PubMed
Guyatt 2008
Hawkey 2006
-
- Hawkey CJ. NSAIDs, coxibs, and the intestine. Journal of Cardiovascular Pharmacology 2006;47 Suppl 1:S72‐5. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horton 2004
-
- Horton R. Vioxx, the implosion of Merck, and aftershocks at the FDA. Lancet 2004;364(9450):1995‐6. - PubMed
Juni 2004
-
- Jüni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta‐analysis. Lancet 2004;364(9450):2021‐9. - PubMed
Loftus 2004
-
- Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004;126(6):1504‐17. - PubMed
MacDonald 1997
Matchaba 2005
-
- Matchaba P, Gitton X, Krammer G, Ehrsam E, Sloan VS, Olson M, et al. Cardiovascular safety of lumiracoxib: a meta‐analysis of all randomized controlled trials > or =1 week and up to 1 year in duration of patients with osteoarthritis and rheumatoid arthritis. Clinical Therapeutics 2005;27(8):1196‐214. - PubMed
Nussmeier 2005
-
- Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX‐2 inhibitors parecoxib and valdecoxib after cardiac surgery. New England Journal of Medicine 2005;352(11):1081‐91. - PubMed
Ott 2003
-
- Ott E, Nussmeier NA, Duke PC, Feneck RO, Alston RP, Snabes MC, et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. Journal of Thoracic and Cardiovascular Surgery 2003;125(6):1481‐92. - PubMed
Pillans 2012
-
- Pillans PI, Ghiculescu RA, Lampe G, Wilson R, Wong R, Macdonald GA. Severe acute liver injury associated with lumiracoxib. Journal of Gastroenterology and Hepatology 2012;27(6):1102‐5. - PubMed
Rankin 1990
-
- Rankin GB. Extraintestinal and systemic manifestations of inflammatory bowel disease. Medical Clinics of North America 1990;74(1):39‐50. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schnitzer 2004
-
- Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004;364(9435):665‐74. - PubMed
Schorr‐Lesnick 1988
-
- Schorr‐Lesnick B, Brandt LJ. Selected rheumatologic and dermatologic manifestations of inflammatory bowel disease. American Journal of Gastroenterology 1988;83(3):216‐23. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Silverstein 1995
-
- Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti‐inflammatory drugs. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1995;123(4):241‐9. - PubMed
Sood 2007
-
- Sood A, Midha V. Epidemiology of inflammatory bowel disease in Asia. Indian Journal of Gastroenterology 2007;26(6):285‐9. - PubMed
Thiéfin 2005
-
- Thiéfin G, Beaugerie L. Toxic effects of nonsteroidal antiinflammatory drugs on the small bowel, colon, and rectum. Joint, Bone, Spine 2005;72(4):286‐94. - PubMed
Vane 1971
-
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin‐like drugs. Nature: New Biology 1971;231(25):232‐5. - PubMed
Ziemer 2007
-
- Ziemer M, Wiesend CL, Vetter R, Weiss J, Blaschke S, Norgauer J, et al. Cutaneous adverse reactions to valdecoxib distinct from Stevens‐Johnson syndrome and toxic epidermal necrolysis. Archives of Dermatology 2007;143(6):711‐6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

