Endoplasmic reticulum stress and cancer
- PMID: 25337575
- PMCID: PMC4204165
- DOI: 10.15430/JCP.2014.19.2.75
Endoplasmic reticulum stress and cancer
Abstract
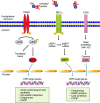
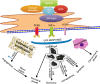
The endoplasmic reticulum (ER) is the principal organelle responsible for multiple cellular functions including protein folding and maturation and the maintenance of cellular homeostasis. ER stress is activated by a variety of factors and triggers the unfolded protein response (UPR), which restores homeostasis or activates cell death. Multiple studies have clarified the link between ER stress and cancer, and particularly the involvement of the UPR. The UPR seems to adjust the paradoxical microenvironment of cancer and, as such, is one of resistance mechanisms against cancer therapy. This review describes the activity of different UPRs involved in tumorigenesis and resistance to cancer therapy.
Keywords: Cancer; Endoplasmic reticulum stress; Unfolded protein response.
Figures
Similar articles
-
Proteostasis In The Endoplasmic Reticulum: Road to Cure.Cancers (Basel). 2019 Nov 14;11(11):1793. doi: 10.3390/cancers11111793. Cancers (Basel). 2019. PMID: 31739582 Free PMC article. Review.
-
Hidden Agenda - The Involvement of Endoplasmic Reticulum Stress and Unfolded Protein Response in Inflammation-Induced Muscle Wasting.Front Immunol. 2022 May 9;13:878755. doi: 10.3389/fimmu.2022.878755. eCollection 2022. Front Immunol. 2022. PMID: 35615361 Free PMC article. Review.
-
Endoplasmic reticulum proteostasis control and gastric cancer.Cancer Lett. 2019 May 1;449:263-271. doi: 10.1016/j.canlet.2019.01.034. Epub 2019 Feb 15. Cancer Lett. 2019. PMID: 30776479 Review.
-
Dual role of Endoplasmic Reticulum Stress-Mediated Unfolded Protein Response Signaling Pathway in Carcinogenesis.Int J Mol Sci. 2019 Sep 5;20(18):4354. doi: 10.3390/ijms20184354. Int J Mol Sci. 2019. PMID: 31491919 Free PMC article. Review.
-
Endoplasmic reticulum stress and lipids in health and diseases.Prog Lipid Res. 2023 Jan;89:101198. doi: 10.1016/j.plipres.2022.101198. Epub 2022 Nov 13. Prog Lipid Res. 2023. PMID: 36379317 Review.
Cited by
-
Unraveling the regulatory role of endoplasmic-reticulum-associated degradation in tumor immunity.Crit Rev Biochem Mol Biol. 2020 Aug;55(4):322-353. doi: 10.1080/10409238.2020.1784085. Epub 2020 Jul 7. Crit Rev Biochem Mol Biol. 2020. PMID: 32633575 Free PMC article. Review.
-
Fenofibrate Improves Insulin Resistance and Hepatic Steatosis and Regulates the Let-7/SERCA2b Axis in High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease Mice.Front Pharmacol. 2022 Jan 19;12:770652. doi: 10.3389/fphar.2021.770652. eCollection 2021. Front Pharmacol. 2022. PMID: 35126113 Free PMC article.
-
Endoplasmic Reticulum as a Therapeutic Target in Cancer: Is there a Role for Flavonoids?Curr Mol Med. 2024;24(3):298-315. doi: 10.2174/1566524023666230320103429. Curr Mol Med. 2024. PMID: 36959143 Review.
-
Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers.Nat Commun. 2019 Dec 12;10(1):5679. doi: 10.1038/s41467-019-13528-0. Nat Commun. 2019. PMID: 31831737 Free PMC article.
-
Anti-tumor Efficacy Assessment of the Sigma Receptor Pan Modulator RC-106. A Promising Therapeutic Tool for Pancreatic Cancer.Front Pharmacol. 2019 May 14;10:490. doi: 10.3389/fphar.2019.00490. eCollection 2019. Front Pharmacol. 2019. PMID: 31156430 Free PMC article.
References
-
- Martinon F. Targeting endoplasmic reticulum signaling pathways in cancer. Acta Oncol. 2012;51:822–30. - PubMed
-
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–58. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
