Osseointegrative properties of electrospun hydroxyapatite-containing nanofibrous chitosan scaffolds
- PMID: 25336062
- PMCID: PMC4356216
- DOI: 10.1089/ten.TEA.2013.0789
Osseointegrative properties of electrospun hydroxyapatite-containing nanofibrous chitosan scaffolds
Abstract
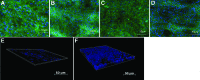
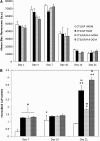
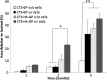
Our long-term goal is to develop smart biomaterials that can facilitate regeneration of critical-size craniofacial lesions. In this study, we tested the hypothesis that biomimetic scaffolds electrospun from chitosan (CTS) will promote tissue repair and regeneration in a critical size calvarial defect. To test this hypothesis, we first compared in vitro ability of electrospun CTS scaffolds crosslinked with genipin (CTS-GP) to those of mineralized CTS-GP scaffolds containing hydroxyapatite (CTS-HA-GP), by assessing proliferation/metabolic activity and alkaline phosphatase (ALP) levels of murine mesenchymal stem cells (mMSCs). The cells' metabolic activity exhibited a biphasic behavior, indicative of initial proliferation followed by subsequent differentiation for all scaffolds. ALP activity of mMSCs, a surrogate measure of osteogenic differentiation, increased over time in culture. After 3 weeks in maintenance medium, ALP activity of mMSCs seeded onto CTS-HA-GP scaffolds was approximately two times higher than that of cells cultured on CTS-GP scaffolds. The mineralized CTS-HA-GP scaffolds were also osseointegrative in vivo, as inferred from the enhanced bone regeneration in a murine model of critical size calvarial defects. Tissue regeneration was evaluated over a 3 month period by microCT and histology (Hematoxylin and Eosin and Masson's Trichrome). Treatment of the lesions with CTS-HA-GP scaffolds induced a 38% increase in the area of de novo generated mineralized tissue area after 3 months, whereas CTS-GP scaffolds only led to a 10% increase. Preseeding with mMSCs significantly enhanced the regenerative capacity of CTS-GP scaffolds (by ∼3-fold), to 35% increase in mineralized tissue area after 3 months. CTS-HA-GP scaffolds preseeded with mMSCs yielded 45% new mineralized tissue formation in the defects. We conclude that the presence of HA in the CTS-GP scaffolds significantly enhances their osseointegrative capacity and that mineralized chitosan-based scaffolds crosslinked with genipin may represent a unique biomaterial with possible clinical relevance for the repair of critical calvarial bone defects.
Figures



Similar articles
-
Electrospun biomimetic scaffold of hydroxyapatite/chitosan supports enhanced osteogenic differentiation of mMSCs.Nanotechnology. 2012 Dec 7;23(48):485102. doi: 10.1088/0957-4484/23/48/485102. Epub 2012 Nov 6. Nanotechnology. 2012. PMID: 23128604
-
The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs.Biomaterials. 2013 Jun;34(18):4404-17. doi: 10.1016/j.biomaterials.2013.02.048. Epub 2013 Mar 17. Biomaterials. 2013. PMID: 23515177
-
Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering.Biomaterials. 2012 Dec;33(36):9167-78. doi: 10.1016/j.biomaterials.2012.09.009. Epub 2012 Sep 27. Biomaterials. 2012. PMID: 23022346 Free PMC article.
-
Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone.Mar Drugs. 2015 Dec 11;13(12):7314-38. doi: 10.3390/md13127068. Mar Drugs. 2015. PMID: 26690453 Free PMC article. Review.
-
Development and application of hydroxyapatite-based scaffolds for bone tissue regeneration: A systematic literature review.Bone. 2024 Jun;183:117075. doi: 10.1016/j.bone.2024.117075. Epub 2024 Mar 18. Bone. 2024. PMID: 38508371 Review.
Cited by
-
Improved cellular infiltration in electrospun fiber via engineered porosity.Tissue Eng. 2007 Sep;13(9):2249-57. doi: 10.1089/ten.2006.0306. Tissue Eng. 2007. PMID: 17536926 Free PMC article.
-
Predifferentiated Gingival Stem Cell-Induced Bone Regeneration in Rat Alveolar Bone Defect Model.Tissue Eng Part A. 2021 Mar;27(5-6):424-436. doi: 10.1089/ten.TEA.2020.0052. Epub 2020 Sep 18. Tissue Eng Part A. 2021. PMID: 32729362 Free PMC article.
-
Silk Fibroin-Alginate-Hydroxyapatite Composite Particles in Bone Tissue Engineering Applications In Vivo.Int J Mol Sci. 2017 Apr 18;18(4):858. doi: 10.3390/ijms18040858. Int J Mol Sci. 2017. PMID: 28420224 Free PMC article.
-
Bone Regeneration Capabilities of Scaffolds Containing Chitosan and Nanometric Hydroxyapatite-Systematic Review Based on In Vivo Examinations.Biomimetics (Basel). 2024 Aug 20;9(8):503. doi: 10.3390/biomimetics9080503. Biomimetics (Basel). 2024. PMID: 39194482 Free PMC article. Review.
-
The role of nanohydroxyapatite on the morphological, physical, and biological properties of chitosan nanofibers.Clin Oral Investig. 2021 May;25(5):3095-3103. doi: 10.1007/s00784-020-03633-6. Epub 2020 Oct 13. Clin Oral Investig. 2021. PMID: 33047204
References
-
- Senagore A.J.The Gale encyclopedia of surgery: a guide for patients and caregivers. Detroit: Gale, 2004.
-
- Laurie S.W., Kaban L.B., Mulliken J.B., and Murray J.E.Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg 73,933, 1984 - PubMed
-
- Kurz L.T., Garfin S.R., and Booth R.E., Jr.Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine (Phila Pa 1976) 14,1324, 1989 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

