LTX-109 is a novel agent for nasal decolonization of methicillin-resistant and -sensitive Staphylococcus aureus
- PMID: 25331699
- PMCID: PMC4291342
- DOI: 10.1128/AAC.03513-14
LTX-109 is a novel agent for nasal decolonization of methicillin-resistant and -sensitive Staphylococcus aureus
Abstract
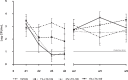
Nasal decolonization has a proven effect on the prevention of severe Staphylococcus aureus infections and the control of methicillin-resistant S. aureus (MRSA). However, rising rates of resistance to antibiotics highlight the need for new substances for nasal decolonization. LTX-109 is a broad-spectrum, fast-acting bactericidal antimicrobial drug for topical treatment, which causes membrane disruption and cell lysis. This mechanism of action is not associated with cross-resistance and has a low propensity for development of resistance. In the present study, persistent nasal MRSA and methicillin-sensitive S. aureus (MSSA) carriers were treated for 3 days with vehicle or with 1%, 2%, or 5% LTX-109. A significant effect on nasal decolonization was observed already after 2 days of LTX-109 treatment in subjects treated with 2% or 5% LTX-109 compared to vehicle (P ≤ 0.0012 by Dunnett's test). No safety issues were noted during the 9-week follow-up period. Minimal reversible epithelial lesions were observed in the nasal cavity. The systemic exposure was very low, with a maximum concentration of drug in plasma (Cmax) at 1 to 2 h postdosing (3.72 to 11.7 ng/ml). One week after treatment initiation, LTX-109 was not detectable in any subject. Intranasal treatment of S. aureus with LTX-109 is safe and reduces the bacterial load already after a single day of treatment. Hence, LTX-109 has potential as a new and effective antimicrobial agent with a low propensity of resistance development that can prevent infections by MSSA/MRSA during hospitalization. (This study has been registered at ClinicalTrials.gov under registration no. NCT01158235.).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Is preoperative staphylococcal decolonization efficient in total joint arthroplasty.J Arthroplasty. 2015 Mar;30(3):444-6. doi: 10.1016/j.arth.2014.10.017. Epub 2014 Oct 23. J Arthroplasty. 2015. PMID: 25453634
-
Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Screening and Decolonization to Reduce Surgical Site Infection in Elective Total Joint Arthroplasty.J Arthroplasty. 2016 Sep;31(9 Suppl):144-7. doi: 10.1016/j.arth.2016.05.019. Epub 2016 May 18. J Arthroplasty. 2016. PMID: 27387479 Clinical Trial.
-
Nasal decolonization of Staphylococcus aureus with mupirocin: strengths, weaknesses and future prospects.J Antimicrob Chemother. 2009 Jul;64(1):9-15. doi: 10.1093/jac/dkp159. Epub 2009 May 18. J Antimicrob Chemother. 2009. PMID: 19451132 Free PMC article. Review.
-
Clearance of nasal Staphylococcus aureus colonization with triple antibiotic ointment.J Drugs Dermatol. 2012 Dec;11(12):1490-2. J Drugs Dermatol. 2012. PMID: 23377521 Clinical Trial.
-
Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00682-20. doi: 10.1128/AAC.00682-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32571829 Free PMC article. Review.
Cited by
-
In vitro activities of LTX-109, a synthetic antimicrobial peptide, against methicillin-resistant, vancomycin-intermediate, vancomycin-resistant, daptomycin-nonsusceptible, and linezolid-nonsusceptible Staphylococcus aureus.Antimicrob Agents Chemother. 2012 Aug;56(8):4478-82. doi: 10.1128/AAC.00194-12. Epub 2012 May 14. Antimicrob Agents Chemother. 2012. PMID: 22585222 Free PMC article.
-
ABC Exporters in Pathogenesis: Role of Synthetic Anti-Microbial Peptides.Protein J. 2020 Dec;39(6):657-670. doi: 10.1007/s10930-020-09931-y. Epub 2020 Oct 17. Protein J. 2020. PMID: 33068233
-
Ameliorating Fibrosis in Murine and Human Tissues with END55, an Endostatin-Derived Fusion Protein Made in Plants.Biomedicines. 2022 Nov 9;10(11):2861. doi: 10.3390/biomedicines10112861. Biomedicines. 2022. PMID: 36359382 Free PMC article.
-
Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections.Front Microbiol. 2018 Oct 8;9:2419. doi: 10.3389/fmicb.2018.02419. eCollection 2018. Front Microbiol. 2018. PMID: 30349525 Free PMC article. Review.
-
Recent Advances in Amphipathic Peptidomimetics as Antimicrobial Agents to Combat Drug Resistance.Molecules. 2024 May 24;29(11):2492. doi: 10.3390/molecules29112492. Molecules. 2024. PMID: 38893366 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

