Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings
- PMID: 25330236
- PMCID: PMC4199509
- DOI: 10.1371/journal.pgen.1004701
Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings
Abstract
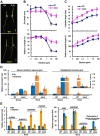
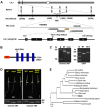
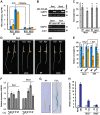
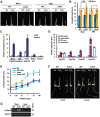
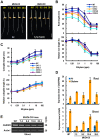
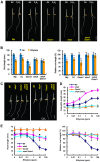
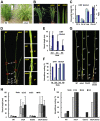
Ethylene and abscisic acid (ABA) have a complicated interplay in many developmental processes. Their interaction in rice is largely unclear. Here, we characterized a rice ethylene-response mutant mhz4, which exhibited reduced ethylene-response in roots but enhanced ethylene-response in coleoptiles of etiolated seedlings. MHZ4 was identified through map-based cloning and encoded a chloroplast-localized membrane protein homologous to Arabidopsis thaliana (Arabidopsis) ABA4, which is responsible for a branch of ABA biosynthesis. MHZ4 mutation reduced ABA level, but promoted ethylene production. Ethylene induced MHZ4 expression and promoted ABA accumulation in roots. MHZ4 overexpression resulted in enhanced and reduced ethylene response in roots and coleoptiles, respectively. In root, MHZ4-dependent ABA pathway acts at or downstream of ethylene receptors and positively regulates root ethylene response. This ethylene-ABA interaction mode is different from that reported in Arabidopsis, where ethylene-mediated root inhibition is independent of ABA function. In coleoptile, MHZ4-dependent ABA pathway acts at or upstream of OsEIN2 to negatively regulate coleoptile ethylene response, possibly by affecting OsEIN2 expression. At mature stage, mhz4 mutation affects branching and adventitious root formation on stem nodes of higher positions, as well as yield-related traits. Together, our findings reveal a novel mode of interplay between ethylene and ABA in control of rice growth and development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Ethylene responses in rice roots and coleoptiles are differentially regulated by a carotenoid isomerase-mediated abscisic acid pathway.Plant Cell. 2015 Apr;27(4):1061-81. doi: 10.1105/tpc.15.00080. Epub 2015 Apr 3. Plant Cell. 2015. PMID: 25841037 Free PMC article.
-
MAOHUZI6/ETHYLENE INSENSITIVE3-LIKE1 and ETHYLENE INSENSITIVE3-LIKE2 Regulate Ethylene Response of Roots and Coleoptiles and Negatively Affect Salt Tolerance in Rice.Plant Physiol. 2015 Sep;169(1):148-65. doi: 10.1104/pp.15.00353. Epub 2015 May 20. Plant Physiol. 2015. PMID: 25995326 Free PMC article.
-
Ethylene-Inhibited Jasmonic Acid Biosynthesis Promotes Mesocotyl/Coleoptile Elongation of Etiolated Rice Seedlings.Plant Cell. 2017 May;29(5):1053-1072. doi: 10.1105/tpc.16.00981. Epub 2017 May 2. Plant Cell. 2017. PMID: 28465411 Free PMC article.
-
Ethylene-mediated regulation of coleoptile elongation in rice seedlings.Plant Cell Environ. 2023 Apr;46(4):1060-1074. doi: 10.1111/pce.14492. Epub 2022 Dec 2. Plant Cell Environ. 2023. PMID: 36397123 Review.
-
ABA signal in rice under stress conditions.Rice (N Y). 2012 Feb 27;5(1):1. doi: 10.1186/1939-8433-5-1. eCollection 2012. Rice (N Y). 2012. PMID: 24764501 Free PMC article. Review.
Cited by
-
Uncovering root compaction response mechanisms: new insights and opportunities.J Exp Bot. 2024 Jan 10;75(2):578-583. doi: 10.1093/jxb/erad389. J Exp Bot. 2024. PMID: 37950742 Free PMC article.
-
Spatiotemporal transcriptomic atlas of rhizome formation in Oryza longistaminata.Plant Biotechnol J. 2024 Jun;22(6):1652-1668. doi: 10.1111/pbi.14294. Epub 2024 Feb 12. Plant Biotechnol J. 2024. PMID: 38345936 Free PMC article.
-
The Arabidopsis transcription factor ABIG1 relays ABA signaled growth inhibition and drought induced senescence.Elife. 2016 Oct 4;5:e13768. doi: 10.7554/eLife.13768. Elife. 2016. PMID: 27697148 Free PMC article.
-
Differentially localized rice ethylene receptors OsERS1 and OsETR2 and their potential role during submergence.Plant Signal Behav. 2017 Aug 3;12(8):e1356532. doi: 10.1080/15592324.2017.1356532. Epub 2017 Jul 31. Plant Signal Behav. 2017. PMID: 28758833 Free PMC article.
-
ABSCISIC ACID-DEFICIENT4 Has an Essential Function in Both cis-Violaxanthin and cis-Neoxanthin Synthesis.Plant Physiol. 2020 Nov;184(3):1303-1316. doi: 10.1104/pp.20.00947. Epub 2020 Sep 3. Plant Physiol. 2020. PMID: 32883757 Free PMC article.
References
-
- Abeles FB, Morgan PW, Saltveit JME (1992) Ethylene in Plant Biology. 2nd ed. (San Diego, CA: Academic Press).
-
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18. - PubMed
-
- Hall B, Shakeel S, Schaller GE (2007) Ethylene receptors: ethylene perception and signal transduction. J Plant Growth Regul 26: 118–130.
-
- Ju C, Chang C (2012) Advances in ethylene signalling: protein complexes at the endoplasmic reticulum membrane. AoB PLANTS 2012: pls031 doi:10.1093/aobpla/pls031 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

