Platelets, acting in part via P-selectin, mediate cytomegalovirus-induced microvascular dysfunction
- PMID: 25326535
- PMCID: PMC4269701
- DOI: 10.1152/ajpheart.00201.2014
Platelets, acting in part via P-selectin, mediate cytomegalovirus-induced microvascular dysfunction
Abstract
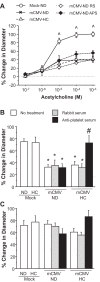
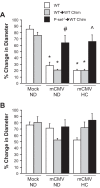
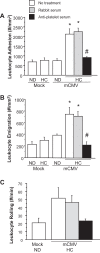
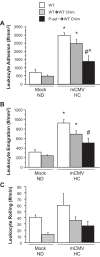
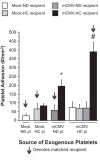
Cytomegalovirus (CMV) infects a majority of the population worldwide. It has been implicated in cardiovascular disease, induces microvascular dysfunction, and synergizes with hypercholesterolemia to promote leukocyte and platelet recruitment in venules. Although platelets and platelet-associated P-selectin contribute to cardiovascular disease inflammation, their role in CMV-induced vascular responses is unknown. We assessed the role of platelets in CMV-induced microvascular dysfunction by depleting platelets and developing bone marrow chimeric mice deficient in platelet P-selectin. Wild-type and chimeric mice received mock or murine (m)CMV intraperitoneally. Five weeks later, some mice were switched to a high-cholesterol diet (HC) to investigate the synergism between mCMV and HC. Arteriolar vasodilation and recruitment of leukocytes and donor platelets in venules were measured at 11wk. mCMV with or without HC caused significant endothelial dysfunction in arterioles. Platelet depletion restored normal vasodilation in mCMV-HC but not mCMV-ND mice, whereas protection was seen in both groups for platelet P-selectin chimeras. Only mCMV + HC elevated leukocyte and platelet recruitment in venules. Leukocyte adhesion was reduced to mock levels by acute platelet depletion but was only partially decreased in platelet P-selectin chimeras. Platelets from mCMV-HC mice and, to a lesser extent, mCMV-ND but not mock-HC mice showed significant adhesion in mCMV-HC recipients. Our findings implicate a role for platelets, acting through P-selectin, in CMV-induced arteriolar dysfunction and suggest that the addition of HC leads to a platelet-dependent, inflammatory infiltrate that is only partly platelet P-selectin dependent. CMV appeared to have a stronger activating influence than HC on platelets and may represent an additional therapeutic target in vulnerable patients.
Keywords: arteriolar dilation; hypercholesterolemia; leukocyte adhesion; microvasculature; platelets.
Copyright © 2014 the American Physiological Society.
Figures

Similar articles
-
P-selectin mediates the microvascular dysfunction associated with persistent cytomegalovirus infection in normocholesterolemic and hypercholesterolemic mice.Microcirculation. 2011 Aug;18(6):452-62. doi: 10.1111/j.1549-8719.2011.00106.x. Microcirculation. 2011. PMID: 21457388 Free PMC article.
-
Cytomegalovirus infection leads to microvascular dysfunction and exacerbates hypercholesterolemia-induced responses.Am J Pathol. 2010 Oct;177(4):2134-44. doi: 10.2353/ajpath.2010.100307. Epub 2010 Aug 27. Am J Pathol. 2010. PMID: 20802174 Free PMC article.
-
NAD(P)H oxidase and eNOS play differential roles in cytomegalovirus infection-induced microvascular dysfunction.Free Radic Biol Med. 2011 Dec 15;51(12):2300-8. doi: 10.1016/j.freeradbiomed.2011.09.039. Epub 2011 Oct 6. Free Radic Biol Med. 2011. PMID: 22033010 Free PMC article.
-
Platelet-leukocyte interactions.Am Heart J. 1998 May;135(5 Pt 2 Su):S146-51. doi: 10.1016/s0002-8703(98)70242-x. Am Heart J. 1998. PMID: 9588393 Review.
-
Inactivation of cytomegalovirus in platelet concentrates using Helinx technology.Semin Hematol. 2001 Oct;38(4 Suppl 11):27-33. doi: 10.1016/s0037-1963(01)90121-0. Semin Hematol. 2001. PMID: 11727283 Review.
Cited by
-
Microvascular dysfunction in pediatric patients with SARS-COV-2 pneumonia: report of three severe cases.Microvasc Res. 2022 May;141:104312. doi: 10.1016/j.mvr.2022.104312. Epub 2022 Jan 10. Microvasc Res. 2022. PMID: 35026289 Free PMC article.
References
-
- Aslam M, Anderson JL, Guglietti D, Cardwell D. CMV-induced neonatal thrombocytopenia: a case report and review of the literature. Am J Perinatol 24: 429–434, 2007. - PubMed
-
- Assinger A, Kral J, Yaiw KC, Schrottmaier W, Kurzejamska E, Wang Y, Mohammad AA, Religa P, Rahbar A, Schabbauer G, Butler LM, Soderberg-Naucler C. Human cytomegalovirus-platelet interaction triggers Toll-like receptor 2-dependent proinflammatory and proangiogenic responses. Arterioscler Thromb Vasc Biol 34: 801–809, 2014. - PubMed
-
- Atzmony L, Halutz O, Avidor B, Finn T, Zimmerman O, Steinvil A, Zeltser D, Giladi M, Justo D. Incidence of cytomegalovirus-associated thrombosis and its risk factors: a case-control study. Thromb Res 126: e439–e443, 2010. - PubMed
-
- Blum A, Giladi M, Weinberg M, Kaplan G, Pasternack H, Laniado S, Miller H. High anti-cytomegalovirus (CMV) IgG antibody titer is associated with coronary artery disease and may predict post-coronary balloon angioplasty restenosis. Am J Cardiol 81: 866–868, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

