Diffusion tensor imaging in Alzheimer's disease: insights into the limbic-diencephalic network and methodological considerations
- PMID: 25324775
- PMCID: PMC4183111
- DOI: 10.3389/fnagi.2014.00266
Diffusion tensor imaging in Alzheimer's disease: insights into the limbic-diencephalic network and methodological considerations
Abstract
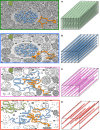
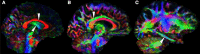
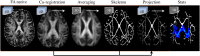
Glucose hypometabolism and gray matter atrophy are well known consequences of Alzheimer's disease (AD). Studies using these measures have shown that the earliest clinical stages, in which memory impairment is a relatively isolated feature, are associated with degeneration in an apparently remote group of areas-mesial temporal lobe (MTL), diencephalic structures such as anterior thalamus and mammillary bodies, and posterior cingulate. These sites are thought to be strongly anatomically inter-connected via a limbic-diencephalic network. Diffusion tensor imaging or DTI-an imaging technique capable of probing white matter tissue microstructure-has recently confirmed degeneration of the white matter connections of the limbic-diencephalic network in AD by way of an unbiased analysis strategy known as tract-based spatial statistics (TBSS). The present review contextualizes the relevance of these findings, in which the fornix is likely to play a fundamental role in linking MTL and diencephalon. An interesting by-product of this work has been in showing that alterations in diffusion behavior are complex in AD-while early studies tended to focus on fractional anisotropy, recent work has highlighted that this measure is not the most sensitive to early changes. Finally, this review will discuss in detail several technical aspects of DTI both in terms of image acquisition and TBSS analysis as both of these factors have important implications to ensure reliable observations are made that inform understanding of neurodegenerative diseases.
Keywords: Alzheimer's disease biomarkers; Alzheimer's disease neurobiology; DTI criteria; axonal loss; circuit of Papez; long association tracts; neurodegenerative diseases; splenium.
Figures
Similar articles
-
Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease.Brain. 2010 Feb;133(Pt 2):529-39. doi: 10.1093/brain/awp257. Epub 2009 Nov 13. Brain. 2010. PMID: 19914928
-
The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review.Neurosurg Focus. 2016 Sep;41(3):E12. doi: 10.3171/2016.6.FOCUS16192. Neurosurg Focus. 2016. PMID: 27581308 Review.
-
Associations between white matter microstructure and amyloid burden in preclinical Alzheimer's disease: A multimodal imaging investigation.Neuroimage Clin. 2014 Feb 19;4:604-14. doi: 10.1016/j.nicl.2014.02.001. eCollection 2014. Neuroimage Clin. 2014. PMID: 24936411 Free PMC article.
-
Fractional anisotropy changes in Alzheimer's disease depend on the underlying fiber tract architecture: a multiparametric DTI study using joint independent component analysis.J Alzheimers Dis. 2014;41(1):69-83. doi: 10.3233/JAD-131829. J Alzheimers Dis. 2014. PMID: 24577476
-
Fractional anisotropy of the fornix and hippocampal atrophy in Alzheimer's disease.Front Aging Neurosci. 2014 Nov 13;6:316. doi: 10.3389/fnagi.2014.00316. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25431558 Free PMC article. Review.
Cited by
-
Thalamic pathology and memory loss in early Alzheimer's disease: moving the focus from the medial temporal lobe to Papez circuit.Brain. 2016 Jul;139(Pt 7):1877-90. doi: 10.1093/brain/aww083. Epub 2016 Apr 28. Brain. 2016. PMID: 27190025 Free PMC article. Review.
-
Neurofilament Light Chain as a Biomarker, and Correlation with Magnetic Resonance Imaging in Diagnosis of CNS-Related Disorders.Mol Neurobiol. 2020 Jan;57(1):469-491. doi: 10.1007/s12035-019-01698-3. Epub 2019 Aug 5. Mol Neurobiol. 2020. PMID: 31385229 Free PMC article. Review.
-
Diffusion tensor tractography of the fornix in cerebral amyloid angiopathy, mild cognitive impairment and Alzheimer's disease.Neuroimage Clin. 2022;34:103002. doi: 10.1016/j.nicl.2022.103002. Epub 2022 Apr 4. Neuroimage Clin. 2022. PMID: 35413649 Free PMC article.
-
White Matter Microstructural Damage as an Early Sign of Subjective Cognitive Decline.Front Aging Neurosci. 2020 Jan 28;11:378. doi: 10.3389/fnagi.2019.00378. eCollection 2019. Front Aging Neurosci. 2020. PMID: 32047428 Free PMC article.
-
Exploring Patterns of Alteration in Alzheimer's Disease Brain Networks: A Combined Structural and Functional Connectomics Analysis.Front Neurosci. 2016 Sep 7;10:380. doi: 10.3389/fnins.2016.00380. eCollection 2016. Front Neurosci. 2016. PMID: 27656119 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

