The E2 ubiquitin-conjugating enzyme UBE2J1 is required for spermiogenesis in mice
- PMID: 25320092
- PMCID: PMC4263858
- DOI: 10.1074/jbc.M114.604132
The E2 ubiquitin-conjugating enzyme UBE2J1 is required for spermiogenesis in mice
Abstract
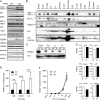
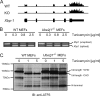
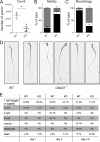
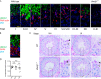
ER-resident proteins destined for degradation are dislocated into the cytosol by components of the ER quality control machinery for proteasomal degradation. Dislocation substrates are ubiquitylated in the cytosol by E2 ubiquitin-conjugating/E3 ligase complexes. UBE2J1 is one of the well-characterized E2 enzymes that participate in this process. However, the physiological function of Ube2j1 is poorly defined. We find that Ube2j1(-/-) mice have reduced viability and fail to thrive early after birth. Male Ube2j1(-/-) mice are sterile due to a defect in late spermatogenesis. Ultrastructural analysis shows that removal of the cytoplasm is incomplete in Ube2j1(-/-) elongating spermatids, compromising the release of mature elongate spermatids into the lumen of the seminiferous tubule. Our findings identify an essential function for the ubiquitin-proteasome-system in spermiogenesis and define a novel, non-redundant physiological function for the dislocation step of ER quality control.
Keywords: ER Quality Control; ERAD Tuning; Endoplasmic Reticulum-associated Protein Degradation (ERAD); Gene Knockout; Male Infertility; Mouse Model; Spermatogenesis; Spermiogenesis; Ubiquitin-conjugating Enzyme (E2 enzyme).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation.Proc Natl Acad Sci U S A. 2011 Feb 1;108(5):2034-9. doi: 10.1073/pnas.1016229108. Epub 2011 Jan 18. Proc Natl Acad Sci U S A. 2011. PMID: 21245296 Free PMC article.
-
The role of ubiquitin-conjugating enzyme Ube2j1 phosphorylation and its degradation by proteasome during endoplasmic stress recovery.J Cell Commun Signal. 2017 Sep;11(3):265-273. doi: 10.1007/s12079-017-0386-6. Epub 2017 Mar 20. J Cell Commun Signal. 2017. PMID: 28321712 Free PMC article.
-
S-Nitrosylation at the active site decreases the ubiquitin-conjugating activity of ubiquitin-conjugating enzyme E2 D1 (UBE2D1), an ERAD-associated protein.Biochem Biophys Res Commun. 2020 Apr 16;524(4):910-915. doi: 10.1016/j.bbrc.2020.02.011. Epub 2020 Feb 10. Biochem Biophys Res Commun. 2020. PMID: 32051088
-
The role of ubiquitin-conjugating enzyme in the process of spermatogenesis.Reprod Biol Endocrinol. 2024 Aug 28;22(1):110. doi: 10.1186/s12958-024-01282-y. Reprod Biol Endocrinol. 2024. PMID: 39198846 Free PMC article. Review.
-
The role of p97/Cdc48p in endoplasmic reticulum-associated degradation: from the immune system to yeast.Curr Top Microbiol Immunol. 2005;300:95-125. doi: 10.1007/3-540-28007-3_5. Curr Top Microbiol Immunol. 2005. PMID: 16573238 Review.
Cited by
-
E3 ubiquitin ligase ASB17 is required for spermiation in mice.Transl Androl Urol. 2021 Dec;10(12):4320-4332. doi: 10.21037/tau-21-789. Transl Androl Urol. 2021. PMID: 35070814 Free PMC article.
-
FBXO24 modulates mRNA alternative splicing and MIWI degradation and is required for normal sperm formation and male fertility.Elife. 2024 Mar 12;12:RP91666. doi: 10.7554/eLife.91666. Elife. 2024. PMID: 38470475 Free PMC article.
-
The testis-specifically expressed gene Trim69 is not essential for fertility in mice.J Biomed Res. 2020 Aug 21;35(1):47-60. doi: 10.7555/JBR.34.20200069. J Biomed Res. 2020. PMID: 33273151 Free PMC article.
-
Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery.Nat Cell Biol. 2016 Nov;18(11):1173-1184. doi: 10.1038/ncb3423. Epub 2016 Oct 17. Nat Cell Biol. 2016. PMID: 27749824
-
New Insights into the Role of E2s in the Pathogenesis of Diseases: Lessons Learned from UBE2O.Mol Cells. 2018 Mar 31;41(3):168-178. doi: 10.14348/molcells.2018.0008. Epub 2018 Mar 20. Mol Cells. 2018. PMID: 29562734 Free PMC article. Review.
References
-
- Lester D., Farquharson C., Russell G., Houston B. (2000) Identification of a family of noncanonical ubiquitin-conjugating enzymes structurally related to yeast UBC6. Biochem. Biophys. Res. Commun. 269, 474–480 - PubMed
-
- Lenk U., Yu H., Walter J., Gelman M. S., Hartmann E., Kopito R. R., Sommer T. (2002) A role for mammalian Ubc6 homologues in ER-associated protein degradation. J. Cell Sci. 115, 3007–3014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

