Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination
- PMID: 25319708
- PMCID: PMC6705285
- DOI: 10.1523/JNEUROSCI.3491-13.2014
Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination
Abstract
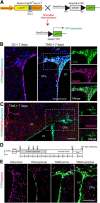
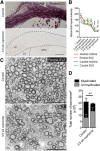
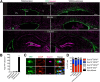
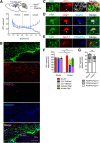
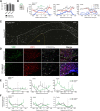
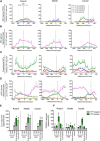
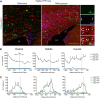
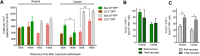
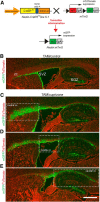
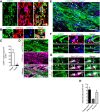
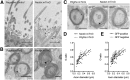
Parenchymal oligodendrocyte progenitor cells (pOPCs) are considered the principal cell type responsible for oligodendrogenesis and remyelinaton in demyelinating diseases. Recent studies have demonstrated that neural precursor cells (NPCs) from the adult subventricular zone (SVZ) can also generate new oligodendrocytes after demyelination. However, the relative contribution of NPCs versus pOPCs to remyelination is unknown. We used in vivo genetic fate mapping to assess the behavior of each progenitor type within the corpus callosi (CCs) of mice subjected to cuprizone-induced demyelination. Nestin-CreER(T2) and Pdgfra-CreER(T2) transgenic mice were crossed with fluorescent Cre reporter strains to map the fate of NPCs and pOPCs respectively. In cuprizone-challenged mice, substantial numbers of NPCs migrated into the demyelinated CC and contributed to oligodendrogenesis. This capacity was most prominent in rostral regions adjacent to the SVZ where NPC-derived oligodendrocytes significantly outnumbered those generated from pOPCs. Sixty-two percent of all nodes of Ranvier in this region were flanked by at least one paranode generated from an NPC-derived oligodendrocyte. Remarkably, g-ratios (ratio of the axon diameter to the diameter of the axon plus myelin sheath) of myelinated axons in regions subject to significant NPC-derived remyelination were equivalent to those of unchallenged controls, and immunoelectron microscopy revealed that NPC-derived myelin was significantly thicker than that generated by pOPCs, regardless of axonal caliber. We also demonstrate that a reduced efficiency of remyelination in the caudal CC was associated with long-term impairment in the maturation of oligodendrogenic NPCs but only transient delay in pOPC differentiation. Collectively, our data define a major distinct role for NPCs in remyelination, identifying them as a key target for enhancing myelin repair in demyelinating diseases.
Keywords: demyelination; multiple sclerosis; myelin; neural precursor cells; oligodendrocyte progenitor cells; remyelination.
Copyright © 2014 the authors 0270-6474/14/3414128-19$15.00/0.
Figures

Similar articles
-
Neural Stem Cells of the Subventricular Zone Contribute to Neuroprotection of the Corpus Callosum after Cuprizone-Induced Demyelination.J Neurosci. 2019 Jul 10;39(28):5481-5492. doi: 10.1523/JNEUROSCI.0227-18.2019. Epub 2019 May 28. J Neurosci. 2019. PMID: 31138656 Free PMC article.
-
Transplanted neural precursors enhance host brain-derived myelin regeneration.J Neurosci. 2009 Dec 16;29(50):15694-702. doi: 10.1523/JNEUROSCI.3364-09.2009. J Neurosci. 2009. PMID: 20016084 Free PMC article.
-
HB-EGF and EGF infusion following CNS demyelination mitigates age-related decline in regeneration of oligodendrocytes from neural precursor cells originating in the ventricular-subventricular zone.bioRxiv [Preprint]. 2024 Feb 28:2024.02.26.582092. doi: 10.1101/2024.02.26.582092. bioRxiv. 2024. PMID: 38529498 Free PMC article. Preprint.
-
The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
-
Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies.Int J Dev Neurosci. 2013 Nov;31(7):692-700. doi: 10.1016/j.ijdevneu.2013.01.004. Epub 2013 Jan 20. Int J Dev Neurosci. 2013. PMID: 23340483 Review.
Cited by
-
NG2-glia and their functions in the central nervous system.Glia. 2015 Aug;63(8):1429-51. doi: 10.1002/glia.22859. Epub 2015 May 24. Glia. 2015. PMID: 26010717 Free PMC article. Review.
-
How to Use the Cuprizone Model to Study De- and Remyelination.Int J Mol Sci. 2024 Jan 24;25(3):1445. doi: 10.3390/ijms25031445. Int J Mol Sci. 2024. PMID: 38338724 Free PMC article. Review.
-
The Current Challenges for Drug Discovery in CNS Remyelination.Int J Mol Sci. 2021 Mar 12;22(6):2891. doi: 10.3390/ijms22062891. Int J Mol Sci. 2021. PMID: 33809224 Free PMC article. Review.
-
Neural stem cells and oligodendrocyte progenitor cells compete for remyelination in the corpus callosum.Front Cell Neurosci. 2023 Jan 26;17:1114781. doi: 10.3389/fncel.2023.1114781. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36779010 Free PMC article.
-
N-Acetylaspartate Drives Oligodendroglial Differentiation via Histone Deacetylase Activation.Cells. 2023 Jul 14;12(14):1861. doi: 10.3390/cells12141861. Cells. 2023. PMID: 37508525 Free PMC article.
References
-
- Cate HS, Sabo JK, Merlo D, Kemper D, Aumann TD, Robinson J, Merson TD, Emery B, Perreau VM, Kilpatrick TJ. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. J Neurochem. 2010;115:11–22. doi: 10.1111/j.1471-4159.2010.06660.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
