Conformational rearrangements in the pro-apoptotic protein, Bax, as it inserts into mitochondria: a cellular death switch
- PMID: 25315775
- PMCID: PMC4239635
- DOI: 10.1074/jbc.M114.593897
Conformational rearrangements in the pro-apoptotic protein, Bax, as it inserts into mitochondria: a cellular death switch
Abstract
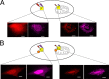
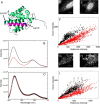
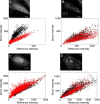
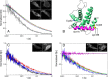
The B-cell lymphoma 2 (Bcl-2) family of proteins regulates the activation of apoptosis through the mitochondria pathway. Pro- and anti-apoptotic members of this family keep each other in check until the correct time to commit to apoptosis. The point of no return for this commitment is the permeabilization of the outer mitochondrial membrane. Translocation of the pro-apoptotic member, Bax, from the cytosol to the mitochondria is the molecular signature of this event. We employed a novel method to reliably detect Förster resonance energy transfer (FRET) between pairs of fluorophores to identify intra-molecular conformational changes and inter-molecular contacts in Bax as this translocation occurs in live cells. In the cytosol, our FRET measurement indicated that the C-terminal helix is exposed instead of tucked away in the core of the protein. In addition fluorescence correlation spectroscopy (FCS) showed that cytosolic Bax diffuses much slower than expected, suggesting possible complex formation or transient membrane interaction. Cross-linking the C-terminal helix (α9) to helix α4 reduced the potential of those interactions to occur. After translocation, our FRET measurements showed that Bax molecules form homo-oligomers in the mitochondria through two distinct interfaces involving the BH3 domain (helix α2) and the C-terminal helix. These findings have implications for possible contacts with other Bcl-2 proteins necessary for the regulation of apoptosis.
Keywords: Apoptosis; B-cell Lymphoma 2 (Bcl-2) Family; Bax; Conformational Change; Fluorescence Correlation Spectroscopy (FCS); Fluorescence Resonance Energy Transfer (FRET).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
The N-terminus and alpha-5, alpha-6 helices of the pro-apoptotic protein Bax, modulate functional interactions with the anti-apoptotic protein Bcl-xL.BMC Cell Biol. 2007 May 23;8:16. doi: 10.1186/1471-2121-8-16. BMC Cell Biol. 2007. PMID: 17519046 Free PMC article.
-
tBid undergoes multiple conformational changes at the membrane required for Bax activation.J Biol Chem. 2013 Jul 26;288(30):22111-27. doi: 10.1074/jbc.M113.482109. Epub 2013 Jun 6. J Biol Chem. 2013. PMID: 23744079 Free PMC article.
-
Synthetic Antibodies Inhibit Bcl-2-associated X Protein (BAX) through Blockade of the N-terminal Activation Site.J Biol Chem. 2016 Jan 1;291(1):89-102. doi: 10.1074/jbc.M115.680918. Epub 2015 Nov 12. J Biol Chem. 2016. PMID: 26565029 Free PMC article.
-
Mitochondria as the target of the pro-apoptotic protein Bax.Biochim Biophys Acta. 2006 Sep-Oct;1757(9-10):1301-11. doi: 10.1016/j.bbabio.2006.05.032. Epub 2006 May 27. Biochim Biophys Acta. 2006. PMID: 16836974 Review.
-
Multistep and multitask Bax activation.Mitochondrion. 2010 Nov;10(6):604-13. doi: 10.1016/j.mito.2010.08.003. Epub 2010 Aug 10. Mitochondrion. 2010. PMID: 20709625 Review.
Cited by
-
Conformational Heterogeneity of Bax Helix 9 Dimer for Apoptotic Pore Formation.Sci Rep. 2016 Jul 6;6:29502. doi: 10.1038/srep29502. Sci Rep. 2016. PMID: 27381287 Free PMC article.
-
Lyciumamide A, a dimer of phenolic amide, protects against NMDA-induced neurotoxicity and potential mechanisms in vitro.J Mol Histol. 2021 Jun;52(3):449-459. doi: 10.1007/s10735-020-09952-y. Epub 2021 Mar 23. J Mol Histol. 2021. PMID: 33755822
-
Robust autoactivation for apoptosis by BAK but not BAX highlights BAK as an important therapeutic target.Cell Death Dis. 2020 Apr 23;11(4):268. doi: 10.1038/s41419-020-2463-7. Cell Death Dis. 2020. PMID: 32327636 Free PMC article.
-
Assembly of Bak homodimers into higher order homooligomers in the mitochondrial apoptotic pore.Sci Rep. 2016 Aug 4;6:30763. doi: 10.1038/srep30763. Sci Rep. 2016. PMID: 27488021 Free PMC article.
-
Disordered clusters of Bak dimers rupture mitochondria during apoptosis.Elife. 2017 Feb 6;6:e19944. doi: 10.7554/eLife.19944. Elife. 2017. PMID: 28182867 Free PMC article.
References
-
- Czabotar P. E., Lessene G., Strasser A., Adams J. M. (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63 - PubMed
-
- Green D. R. (2004) The pathophysiology of mitochondrial cell death. Science 305, 626–629 - PubMed
-
- Cory S., Adams J. M. (2002) The bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2, 647–656 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

