A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration
- PMID: 25312291
- PMCID: PMC4234693
- DOI: 10.1007/s00441-014-2010-x
A review of crosstalk between MAPK and Wnt signals and its impact on cartilage regeneration
Abstract
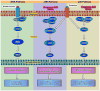
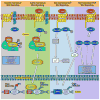
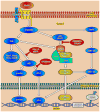
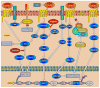
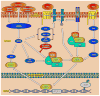
Chondrogenesis is a developmental process that is controlled and coordinated by many growth and differentiation factors, in addition to environmental factors that initiate or suppress cellular signaling pathways and the transcription of specific genes in a temporal-spatial manner. As key signaling molecules in regulating cell proliferation, homeostasis and development, both mitogen-activated protein kinases (MAPK) and the Wnt family participate in morphogenesis and tissue patterning, playing important roles in skeletal development, especially chondrogenesis. Recent findings suggest that both signals are also actively involved in arthritis and related diseases. Despite the implication that crosstalk between MAPK and Wnt signaling has a significant function in cancer, few studies have summarized this interaction and its regulation of chondrogenesis. In this review, we focus on MAPK and Wnt signaling, referencing their relationships in various types of cells and particularly to their influence on chondrogenesis and cartilage development. We also discuss the interactions between MAPK and Wnt signaling with respect to cartilage-related diseases such as osteoarthritis and explore potential therapeutic targets for disease treatments.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
Similar articles
-
Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk.J Biol Chem. 2003 Oct 17;278(42):41227-36. doi: 10.1074/jbc.M305312200. Epub 2003 Jul 31. J Biol Chem. 2003. PMID: 12893825
-
The Key Role of Canonical Wnt/β-catenin Signaling in Cartilage Chondrocytes.Curr Drug Targets. 2016;17(4):475-84. doi: 10.2174/1389450116666150825112623. Curr Drug Targets. 2016. PMID: 26302804 Review.
-
AP-1 transcription factor complex is a target of signals from both WnT-7a and N-cadherin-dependent cell-cell adhesion complex during the regulation of limb mesenchymal chondrogenesis.Exp Cell Res. 2002 Feb 15;273(2):197-203. doi: 10.1006/excr.2001.5448. Exp Cell Res. 2002. PMID: 11822875
-
Cartilage development and degeneration: a Wnt Wnt situation.Cell Biochem Funct. 2012 Dec;30(8):633-42. doi: 10.1002/cbf.2852. Epub 2012 Jun 19. Cell Biochem Funct. 2012. PMID: 22714865 Review.
-
Lung development requires an active ERK/MAPK pathway in the lung mesenchyme.Dev Dyn. 2017 Jan;246(1):72-82. doi: 10.1002/dvdy.24464. Epub 2016 Nov 17. Dev Dyn. 2017. PMID: 27748998
Cited by
-
Long-term dynamic compression enhancement TGF-β3-induced chondrogenesis in bovine stem cells: a gene expression analysis.BMC Genom Data. 2021 Mar 20;22(1):13. doi: 10.1186/s12863-021-00967-2. BMC Genom Data. 2021. PMID: 33743603 Free PMC article.
-
Identification and Functional Annotation of Genes Related to Bone Stability in Laying Hens Using Random Forests.Genes (Basel). 2021 May 8;12(5):702. doi: 10.3390/genes12050702. Genes (Basel). 2021. PMID: 34066823 Free PMC article.
-
Wnt10b protects cardiomyocytes against doxorubicin-induced cell death via MAPK modulation.PLoS One. 2023 Oct 19;18(10):e0277747. doi: 10.1371/journal.pone.0277747. eCollection 2023. PLoS One. 2023. PMID: 37856516 Free PMC article.
-
Intermittent Administration of Parathyroid Hormone Enhances Odonto/Osteogenic Differentiation of Stem Cells from the Apical Papilla via JNK and P38 MAPK Pathways.Stem Cells Int. 2020 Feb 14;2020:5128128. doi: 10.1155/2020/5128128. eCollection 2020. Stem Cells Int. 2020. PMID: 32148520 Free PMC article.
-
Inflammation Responses to Bone Scaffolds under Mechanical Stimuli in Bone Regeneration.J Funct Biomater. 2023 Mar 21;14(3):169. doi: 10.3390/jfb14030169. J Funct Biomater. 2023. PMID: 36976093 Free PMC article. Review.
References
-
- Badger AM, Griswold DE, Kapadia R, Blake S, Swift BA, Hoffman SJ, Stroup GB, Webb E, Rieman DJ, Gowen M, Boehm JC, Adams JL, Lee JC. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:175–183. - PubMed
-
- Beier F, LuValle P. Serum induction of the collagen X promoter requires the Raf/MEK/ERK and p38 pathways. Biochem Biophys Res Commun. 1999;262:50–54. - PubMed
-
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. - PubMed
-
- Bikkavilli RK, Feigin ME, Malbon CC. G alpha o mediates WNT-JNK signaling through dishevelled 1 and 3, RhoA family members, and MEKK 1 and 4 in mammalian cells. J Cell Sci. 2008a;121:234–245. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

