Molecular profile of cochlear immunity in the resident cells of the organ of Corti
- PMID: 25311735
- PMCID: PMC4198756
- DOI: 10.1186/s12974-014-0173-8
Molecular profile of cochlear immunity in the resident cells of the organ of Corti
Abstract
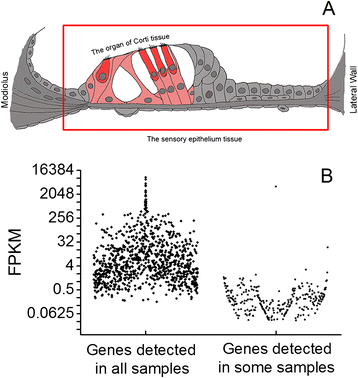
Background: The cochlea is the sensory organ of hearing. In the cochlea, the organ of Corti houses sensory cells that are susceptible to pathological insults. While the organ of Corti lacks immune cells, it does have the capacity for immune activity. We hypothesized that resident cells in the organ of Corti were responsible for the stress-induced immune response of the organ of Corti. This study profiled the molecular composition of the immune system in the organ of Corti and examined the immune response of non-immune epithelial cells to acoustic overstimulation.
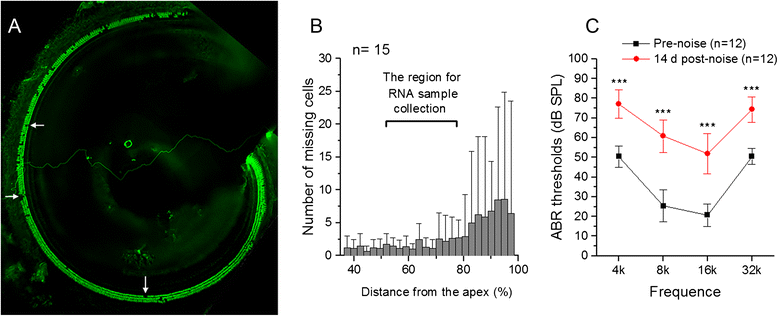
Methods: Using high-throughput RNA-sequencing and qRT-PCR arrays, we identified immune- and inflammation-related genes in both the cochlear sensory epithelium and the organ of Corti. Using bioinformatics analyses, we cataloged the immune genes expressed. We then examined the response of these genes to acoustic overstimulation and determined how changes in immune gene expression were related to sensory cell damage.
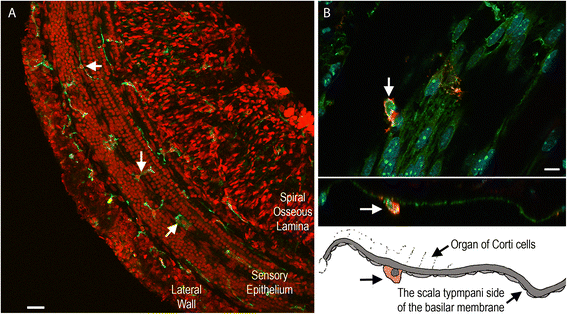
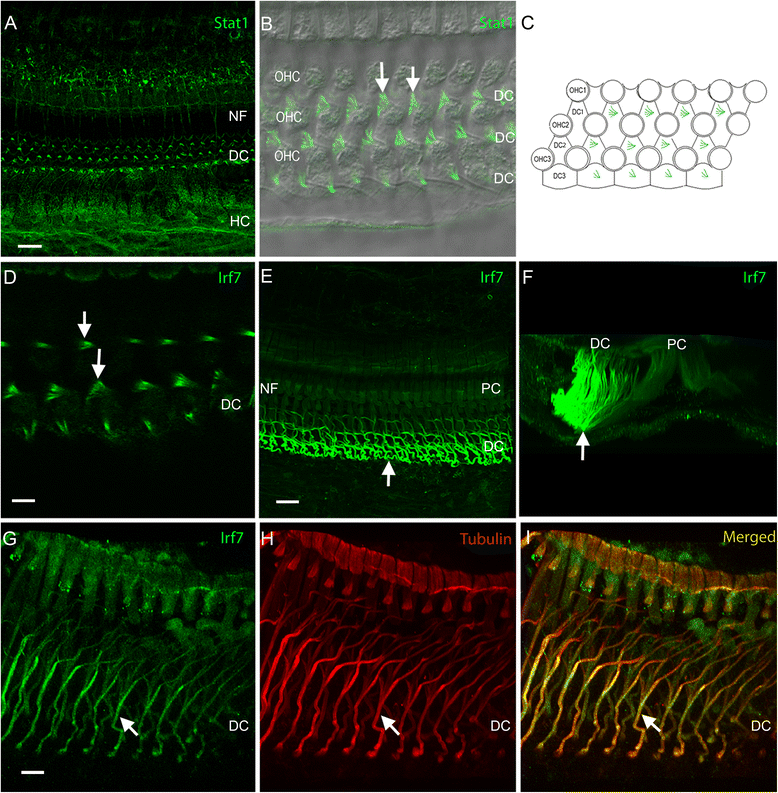
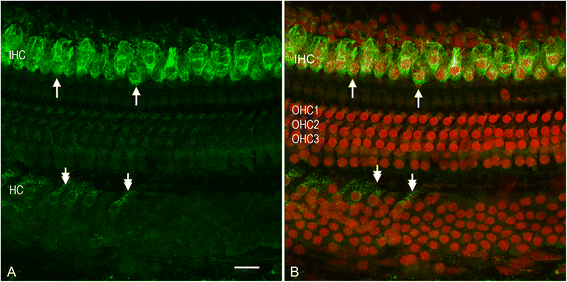
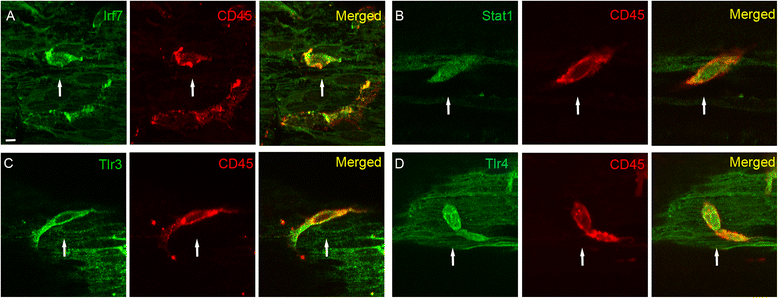
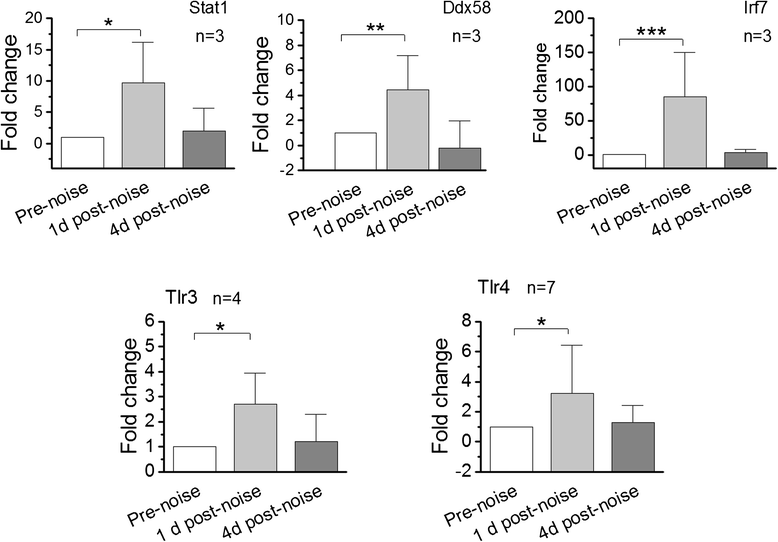
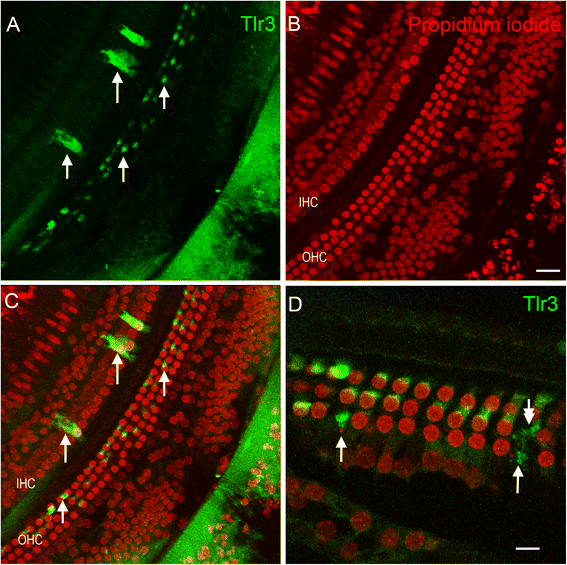
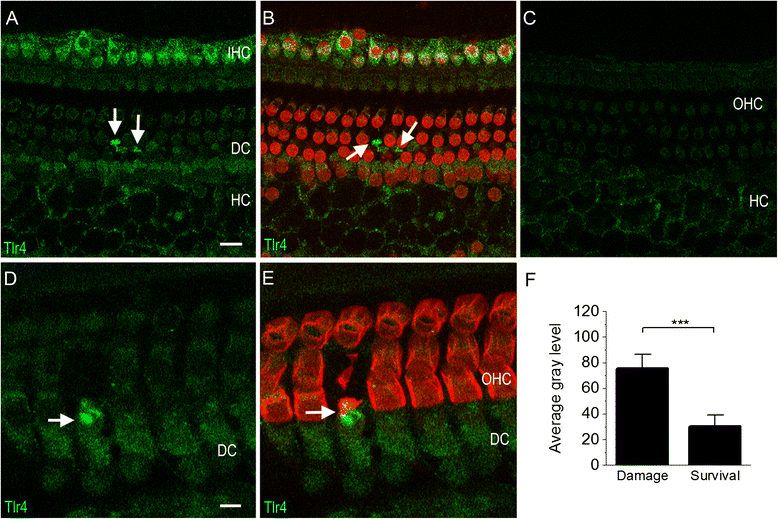
Results: The RNA-sequencing analysis reveals robust expression of immune-related genes in the cochlear sensory epithelium. The qRT-PCR array analysis confirms that many of these genes are constitutively expressed in the resident cells of the organ of Corti. Bioinformatics analyses reveal that the genes expressed are linked to the Toll-like receptor signaling pathway. We demonstrate that expression of Toll-like receptor signaling genes is predominantly from the supporting cells in the organ of Corti cells. Importantly, our data demonstrate that these Toll-like receptor pathway genes are able to respond to acoustic trauma and that their expression changes are associated with sensory cell damage.
Conclusion: The cochlear resident cells in the organ of Corti have immune capacity and participate in the cochlear immune response to acoustic overstimulation.
Figures
Similar articles
-
Variation analysis of transcriptome changes reveals cochlear genes and their associated functions in cochlear susceptibility to acoustic overstimulation.Hear Res. 2015 Dec;330(Pt A):78-89. doi: 10.1016/j.heares.2015.04.010. Epub 2015 May 27. Hear Res. 2015. PMID: 26024952 Free PMC article.
-
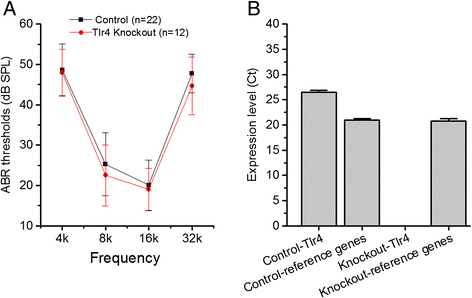
Toll-like receptor 4 modulates the cochlear immune response to acoustic injury.Cell Death Dis. 2016 Jun 2;7(6):e2245. doi: 10.1038/cddis.2016.156. Cell Death Dis. 2016. PMID: 27253409 Free PMC article.
-
Metalloproteinases and their associated genes contribute to the functional integrity and noise-induced damage in the cochlear sensory epithelium.J Neurosci. 2012 Oct 24;32(43):14927-41. doi: 10.1523/JNEUROSCI.1588-12.2012. J Neurosci. 2012. PMID: 23100416 Free PMC article.
-
Genetic influences on susceptibility of the auditory system to aging and environmental factors.Scand Audiol Suppl. 1992;36:1-39. Scand Audiol Suppl. 1992. PMID: 1488615 Review.
-
Purinergic signaling in the organ of Corti: Potential therapeutic targets of sensorineural hearing losses.Brain Res Bull. 2019 Sep;151:109-118. doi: 10.1016/j.brainresbull.2019.01.029. Epub 2019 Feb 2. Brain Res Bull. 2019. PMID: 30721767 Review.
Cited by
-
Visualization of macrophage subsets in the development of the fetal human inner ear.Front Immunol. 2022 Sep 9;13:965196. doi: 10.3389/fimmu.2022.965196. eCollection 2022. Front Immunol. 2022. PMID: 36159857 Free PMC article.
-
Systematic Transcriptome Analysis of Noise-Induced Hearing Loss Pathogenesis Suggests Inflammatory Activities and Multiple Susceptible Molecules and Pathways.Front Genet. 2020 Aug 28;11:968. doi: 10.3389/fgene.2020.00968. eCollection 2020. Front Genet. 2020. PMID: 33005175 Free PMC article.
-
Oncomodulin (OCM) uniquely regulates calcium signaling in neonatal cochlear outer hair cells.Cell Calcium. 2022 Jul;105:102613. doi: 10.1016/j.ceca.2022.102613. Epub 2022 Jun 24. Cell Calcium. 2022. PMID: 35797824 Free PMC article.
-
Distribution of Immune Cells Including Macrophages in the Human Cochlea.Front Neurol. 2021 Nov 22;12:781702. doi: 10.3389/fneur.2021.781702. eCollection 2021. Front Neurol. 2021. PMID: 34880828 Free PMC article. Review.
-
Pioglitazone Ameliorates Gentamicin Ototoxicity by Affecting the TLR and STAT Pathways in the Early Postnatal Organ of Corti.Front Cell Neurosci. 2020 Oct 29;14:566148. doi: 10.3389/fncel.2020.566148. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33192313 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

