A three-dimensional human neural cell culture model of Alzheimer's disease
- PMID: 25307057
- PMCID: PMC4366007
- DOI: 10.1038/nature13800
A three-dimensional human neural cell culture model of Alzheimer's disease
Abstract
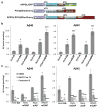
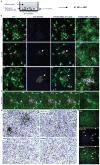
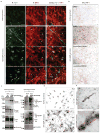
Alzheimer's disease is the most common form of dementia, characterized by two pathological hallmarks: amyloid-β plaques and neurofibrillary tangles. The amyloid hypothesis of Alzheimer's disease posits that the excessive accumulation of amyloid-β peptide leads to neurofibrillary tangles composed of aggregated hyperphosphorylated tau. However, to date, no single disease model has serially linked these two pathological events using human neuronal cells. Mouse models with familial Alzheimer's disease (FAD) mutations exhibit amyloid-β-induced synaptic and memory deficits but they do not fully recapitulate other key pathological events of Alzheimer's disease, including distinct neurofibrillary tangle pathology. Human neurons derived from Alzheimer's disease patients have shown elevated levels of toxic amyloid-β species and phosphorylated tau but did not demonstrate amyloid-β plaques or neurofibrillary tangles. Here we report that FAD mutations in β-amyloid precursor protein and presenilin 1 are able to induce robust extracellular deposition of amyloid-β, including amyloid-β plaques, in a human neural stem-cell-derived three-dimensional (3D) culture system. More importantly, the 3D-differentiated neuronal cells expressing FAD mutations exhibited high levels of detergent-resistant, silver-positive aggregates of phosphorylated tau in the soma and neurites, as well as filamentous tau, as detected by immunoelectron microscopy. Inhibition of amyloid-β generation with β- or γ-secretase inhibitors not only decreased amyloid-β pathology, but also attenuated tauopathy. We also found that glycogen synthase kinase 3 (GSK3) regulated amyloid-β-mediated tau phosphorylation. We have successfully recapitulated amyloid-β and tau pathology in a single 3D human neural cell culture system. Our unique strategy for recapitulating Alzheimer's disease pathology in a 3D neural cell culture model should also serve to facilitate the development of more precise human neural cell models of other neurodegenerative disorders.
Figures
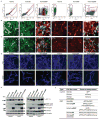
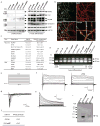
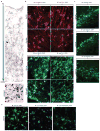
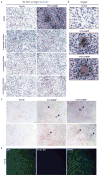
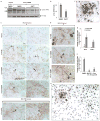
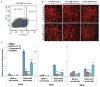
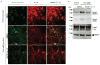

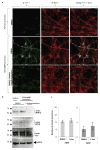

Comment in
-
Neurodegenerative disease: new dimensions in Alzheimer's modelling.Nat Rev Neurosci. 2014 Dec;15(12):765. doi: 10.1038/nrn3860. Epub 2014 Oct 30. Nat Rev Neurosci. 2014. PMID: 25354484 No abstract available.
-
Neurodegenerative disease: New dimensions in Alzheimer's modelling.Nat Rev Drug Discov. 2014 Dec;13(12):887. doi: 10.1038/nrd4494. Nat Rev Drug Discov. 2014. PMID: 25435212 No abstract available.
Similar articles
-
Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation.Alzheimers Res Ther. 2017 Dec 1;9(1):90. doi: 10.1186/s13195-017-0317-z. Alzheimers Res Ther. 2017. PMID: 29191219 Free PMC article.
-
A 3D human neural cell culture system for modeling Alzheimer's disease.Nat Protoc. 2015 Jul;10(7):985-1006. doi: 10.1038/nprot.2015.065. Epub 2015 Jun 11. Nat Protoc. 2015. PMID: 26068894 Free PMC article.
-
Amyloid-induced neurofibrillary tangle formation in Alzheimer's disease: insight from transgenic mouse and tissue-culture models.Int J Dev Neurosci. 2004 Nov;22(7):453-65. doi: 10.1016/j.ijdevneu.2004.07.013. Int J Dev Neurosci. 2004. PMID: 15465275 Review.
-
Aluminum and Neurofibrillary Tangle Co-Localization in Familial Alzheimer's Disease and Related Neurological Disorders.J Alzheimers Dis. 2020;78(1):139-149. doi: 10.3233/JAD-200838. J Alzheimers Dis. 2020. PMID: 32925074 Free PMC article.
-
Exploring the Role of Aggregated Proteomes in the Pathogenesis of Alzheimer's Disease.Curr Protein Pept Sci. 2020;21(12):1164-1173. doi: 10.2174/1389203721666200921152246. Curr Protein Pept Sci. 2020. PMID: 32957903 Review.
Cited by
-
Comprehensive Characterization of CK1δ-Mediated Tau Phosphorylation in Alzheimer's Disease.Front Mol Biosci. 2022 Jun 27;9:872171. doi: 10.3389/fmolb.2022.872171. eCollection 2022. Front Mol Biosci. 2022. PMID: 36203870 Free PMC article.
-
Genetic studies of quantitative MCI and AD phenotypes in ADNI: Progress, opportunities, and plans.Alzheimers Dement. 2015 Jul;11(7):792-814. doi: 10.1016/j.jalz.2015.05.009. Alzheimers Dement. 2015. PMID: 26194313 Free PMC article. Review.
-
Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer's Disease Phenotypes.PLoS One. 2016 Sep 13;11(9):e0161969. doi: 10.1371/journal.pone.0161969. eCollection 2016. PLoS One. 2016. PMID: 27622770 Free PMC article.
-
Herpes Simplex Virus Infection Increases Beta-Amyloid Production and Induces the Development of Alzheimer's Disease.Biomed Res Int. 2022 Aug 31;2022:8804925. doi: 10.1155/2022/8804925. eCollection 2022. Biomed Res Int. 2022. Retraction in: Biomed Res Int. 2023 Dec 29;2023:9806907. doi: 10.1155/2023/9806907 PMID: 36093396 Free PMC article. Retracted.
-
Drug development in Alzheimer's disease: the path to 2025.Alzheimers Res Ther. 2016 Sep 20;8:39. doi: 10.1186/s13195-016-0207-9. Alzheimers Res Ther. 2016. PMID: 27646601 Free PMC article. Review.
References
-
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Selkoe D. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
-
- Duff K. Transgenic mouse models of Alzheimer’s disease: phenotype and mechanisms of pathogenesis. Biochem Soc Symp. 2001;67:195–202. - PubMed
-
- Chin J. Selecting a mouse model of Alzheimer’s disease. Methods Mol Biol. 2011;670:169–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS045860/NS/NINDS NIH HHS/United States
- RF1 AG048080/AG/NIA NIH HHS/United States
- P01 AG015379/AG/NIA NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- P30 NS045776/NS/NINDS NIH HHS/United States
- R01 AG014713/AG/NIA NIH HHS/United States
- 5R37MH060009/MH/NIMH NIH HHS/United States
- R21 AG031483/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- 5P01AG15379/AG/NIA NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- P01 AG004953/AG/NIA NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

