GnRH, anosmia and hypogonadotropic hypogonadism--where are we?
- PMID: 25306902
- PMCID: PMC4703044
- DOI: 10.1016/j.yfrne.2014.09.004
GnRH, anosmia and hypogonadotropic hypogonadism--where are we?
Abstract
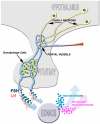
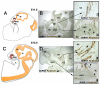
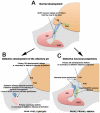
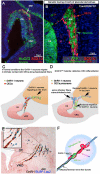
Gonadotropin releasing hormone (GnRH) neurons originate the nasal placode and migrate into the brain during prenatal development. Once within the brain, these cells become integral components of the hypothalamic-pituitary-gonadal axis, essential for reproductive function. Disruption of this system causes hypogonadotropic hypogonadism (HH). HH associated with anosmia is clinically defined as Kallman syndrome (KS). Recent work examining the developing nasal region has shed new light on cellular composition, cell interactions and molecular cues responsible for the development of this system in different species. This review discusses some developmental aspects, animal models and current advancements in our understanding of pathologies affecting GnRH. In addition we discuss how development of neural crest derivatives such as the glia of the olfactory system and craniofacial structures control GnRH development and reproductive function.
Keywords: Craniofacial defects; GnRH; Hypogonadotropic hypogonadism; Hypothalamic–pituitary–gonadal (HPG) axis; Kallmann syndrome; Neural crest; Olfactory ensheathing cells; Olfactory placode; Waardenburg syndrome.
Published by Elsevier Inc.
Figures



Similar articles
-
Gonadotropin-releasing hormone immunoreactivity in the nasal epithelia of adults with Kallmann's syndrome and isolated hypogonadotropic hypogonadism and in the early midtrimester human fetus.J Clin Endocrinol Metab. 1997 Jan;82(1):309-14. doi: 10.1210/jcem.82.1.3673. J Clin Endocrinol Metab. 1997. PMID: 8989279
-
Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism.Proc Natl Acad Sci U S A. 2007 Oct 30;104(44):17447-52. doi: 10.1073/pnas.0707173104. Epub 2007 Oct 24. Proc Natl Acad Sci U S A. 2007. PMID: 17959774 Free PMC article.
-
Mutations in FEZF1 cause Kallmann syndrome.Am J Hum Genet. 2014 Sep 4;95(3):326-31. doi: 10.1016/j.ajhg.2014.08.006. Am J Hum Genet. 2014. PMID: 25192046 Free PMC article.
-
Clinical manifestations of impaired GnRH neuron development and function.Neurosignals. 2008;16(2-3):165-82. doi: 10.1159/000111561. Epub 2008 Feb 5. Neurosignals. 2008. PMID: 18253056 Review.
-
Gonadotropin-releasing hormone neuronal migration.Semin Reprod Med. 2007 Sep;25(5):305-12. doi: 10.1055/s-2007-984736. Semin Reprod Med. 2007. PMID: 17710726 Review.
Cited by
-
Neuropilin-1 expression in GnRH neurons regulates prepubertal weight gain and sexual attraction.EMBO J. 2020 Oct 1;39(19):e104633. doi: 10.15252/embj.2020104633. Epub 2020 Aug 5. EMBO J. 2020. PMID: 32761635 Free PMC article.
-
SOX10 Mutation Screening for 117 Patients with Kallmann Syndrome.J Endocr Soc. 2021 Mar 30;5(7):bvab056. doi: 10.1210/jendso/bvab056. eCollection 2021 Jul 1. J Endocr Soc. 2021. PMID: 34095692 Free PMC article.
-
What do we learn from the murine Jacob/Nsmf gene knockout for human disease?Rare Dis. 2016 Sep 30;4(1):e1241361. doi: 10.1080/21675511.2016.1241361. eCollection 2016. Rare Dis. 2016. PMID: 27803842 Free PMC article.
-
Haploinsufficiency of Homeodomain Proteins Six3, Vax1, and Otx2 Causes Subfertility in Mice via Distinct Mechanisms.Neuroendocrinology. 2019;109(3):200-207. doi: 10.1159/000494086. Epub 2018 Sep 27. Neuroendocrinology. 2019. PMID: 30261489 Free PMC article. Review.
-
Leukemia Inhibitory Factor Represses GnRH Gene Expression via cFOS during Inflammation in Male Mice.Neuroendocrinology. 2019;108(4):291-307. doi: 10.1159/000496754. Epub 2019 Jan 10. Neuroendocrinology. 2019. PMID: 30630179 Free PMC article.
References
-
- Sower SA, Freamat M, Kavanaugh SI. The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: new insights from lampreys. General and comparative endocrinology. 2009;161:20–9. - PubMed
-
- Campbell RK, Satoh N, Degnan BM. Piecing together evolution of the vertebrate endocrine system. Trends Genet. 2004;20:359–66. - PubMed
-
- Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain research. Molecular brain research. 1989;6:311–26. - PubMed
-
- Quinton R, Hasan W, Grant W, Thrasivoulou C, Quiney RE, Besser GM, Bouloux PM. Gonadotropin-releasing hormone immunoreactivity in the nasal epithelia of adults with Kallmann's syndrome and isolated hypogonadotropic hypogonadism and in the early midtrimester human fetus. The Journal of clinical endocrinology and metabolism. 1997;82:309–14. - PubMed
-
- Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol. 2002;23:292–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

