Synaptojanin 1 mutation in Parkinson's disease brings further insight into the neuropathological mechanisms
- PMID: 25302295
- PMCID: PMC4181773
- DOI: 10.1155/2014/289728
Synaptojanin 1 mutation in Parkinson's disease brings further insight into the neuropathological mechanisms
Abstract
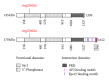
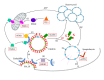
Synaptojanin 1 (SYNJ1) is a phosphoinositide phosphatase highly expressed in nerve terminals. Its two phosphatase domains dephosphorylate phosphoinositides present in membranes, while its proline-rich domain directs protein-protein interactions with synaptic components, leading to efficient recycling of synaptic vesicles in neurons. Triplication of SYNJ1 in Down's syndrome is responsible for higher level of phosphoinositides, enlarged endosomes, and learning deficits. SYNJ1 downregulation in Alzheimer's disease models is protective towards amyloid-beta peptide (Aβ) toxicity. One missense mutation in one of SYNJ1 functional domains was recently incriminated in an autosomal recessive form of early-onset Parkinson's disease (PD). In the third decade of life, these patients develop progressive Parkinsonism with bradykinesia, dystonia, and variable atypical symptoms such as cognitive decline, seizures, and eyelid apraxia. The identification of this new gene, together with the fact that most of the known PD proteins play a role in synaptic vesicle recycling and lipid metabolism, points out that synaptic maintenance is a key player in PD pathological mechanisms. Studying PD genes as a network regulating synaptic activity could bring insight into understanding the neuropathological processes of PD and help identify new genes at fault in this devastating disorder.
Figures
Similar articles
-
Mini-review: Synaptojanin 1 and its implications in membrane trafficking.Neurosci Lett. 2021 Nov 20;765:136288. doi: 10.1016/j.neulet.2021.136288. Epub 2021 Oct 9. Neurosci Lett. 2021. PMID: 34637856 Free PMC article. Review.
-
A Novel SYNJ1 Mutation in a Tunisian Family with Juvenile Parkinson's Disease Associated with Epilepsy.J Mol Neurosci. 2018 Oct;66(2):273-278. doi: 10.1007/s12031-018-1167-2. Epub 2018 Sep 5. J Mol Neurosci. 2018. PMID: 30187305
-
Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism.Hum Mutat. 2013 Sep;34(9):1208-15. doi: 10.1002/humu.22373. Epub 2013 Aug 6. Hum Mutat. 2013. PMID: 23804577
-
Mutational analysis of SYNJ1 gene (PARK20) in Parkinson's disease in a Taiwanese population.Neurobiol Aging. 2015 Oct;36(10):2905.e7-8. doi: 10.1016/j.neurobiolaging.2015.06.009. Epub 2015 Jun 12. Neurobiol Aging. 2015. PMID: 26149920
-
Genetics of Parkinson's disease--state of the art, 2013.Parkinsonism Relat Disord. 2014 Jan;20 Suppl 1:S23-8. doi: 10.1016/S1353-8020(13)70009-9. Parkinsonism Relat Disord. 2014. PMID: 24262182 Review.
Cited by
-
Network pharmacology and molecular docking of endogenous active metabolites in diabetic kidney disease.Ren Fail. 2023;45(2):2290927. doi: 10.1080/0886022X.2023.2290927. Epub 2023 Dec 28. Ren Fail. 2023. PMID: 38152048 Free PMC article.
-
Presynaptic disorders: a clinical and pathophysiological approach focused on the synaptic vesicle.J Inherit Metab Dis. 2018 Nov;41(6):1131-1145. doi: 10.1007/s10545-018-0230-z. Epub 2018 Jul 18. J Inherit Metab Dis. 2018. PMID: 30022305
-
Mini-review: Synaptojanin 1 and its implications in membrane trafficking.Neurosci Lett. 2021 Nov 20;765:136288. doi: 10.1016/j.neulet.2021.136288. Epub 2021 Oct 9. Neurosci Lett. 2021. PMID: 34637856 Free PMC article. Review.
-
Genetic Determinants of Parkinson's Disease: Can They Help to Stratify the Patients Based on the Underlying Molecular Defect?Front Aging Neurosci. 2017 Feb 10;9:20. doi: 10.3389/fnagi.2017.00020. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28239348 Free PMC article. Review.
-
Clinical Variability of SYNJ1-Associated Early-Onset Parkinsonism.Front Neurol. 2021 Mar 25;12:648457. doi: 10.3389/fneur.2021.648457. eCollection 2021. Front Neurol. 2021. PMID: 33841314 Free PMC article.
References
-
- McPherson PS, Takei K, Schmid SL, de Camilli P. p145, a major Grb2-binding protein in brain, is co-localized with dynamin in nerve terminals where it undergoes activity-dependent dephosphorylation. The Journal of Biological Chemistry. 1994;269(48):30132–30139. - PubMed
-
- Quadri M, Fang M, Picillo M, et al. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset parkinsonism. Human Mutation. 2013;34(9):1208–1215. - PubMed
-
- Cremona O, Nimmakayalu M, Haffner C, Bray-Ward P, Ward DC, de Camilli PP. Assignment1 of SYNJ1 to human chromosome 21q22.2 and Synj12 to the murine homologous region on chromosome 16C3-4 by in situ hybridization. Cytogenetics and Cell Genetics. 2000;88(1-2):89–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

