Therapeutic targeting of cellular metabolism in cells with hyperactive mTORC1: a paradigm shift
- PMID: 25298408
- PMCID: PMC4312527
- DOI: 10.1158/1541-7786.MCR-14-0343
Therapeutic targeting of cellular metabolism in cells with hyperactive mTORC1: a paradigm shift
Abstract
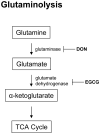
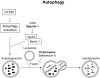
mTORC1 is an established master regulator of cellular metabolic homeostasis, via multiple mechanisms that include altered glucose and glutamine metabolism, and decreased autophagy. mTORC1 is hyperactive in the human disease tuberous sclerosis complex (TSC), an autosomal dominant disorder caused by germline mutations in the TSC1 or TSC2 gene. In TSC-deficient cells, metabolic wiring is extensively disrupted and rerouted as a consequence of mTORC1 hyperactivation, leading to multiple vulnerabilities, including "addiction" to glutamine, glucose, and autophagy. There is synergy between two rapidly evolving trajectories: elucidating the metabolic vulnerabilities of TSC-associated tumor cells, and the development of therapeutic agents that selectively target cancer-associated metabolic defects. The current review focuses on recent work supporting the targeting of cellular metabolic dysregulation for the treatment of tumors in TSC, with relevance to the many other human neoplasms with mTORC1 hyperactivation. These data expose a fundamental paradox in the therapeutic targeting of tumor cells with hyperactive mTORC1: inhibition of mTORC1 may not represent the optimal therapeutic strategy. Inhibiting mTORC1 "fixes" the metabolic vulnerabilities, results in a cytostatic response, and closes the door to metabolic targeting. In contrast, leaving mTORC1 active allows the metabolic vulnerabilities to be targeted with the potential for a cytocidal cellular response. The insights provided here suggest that therapeutic strategies for TSC and other tumors with activation of mTORC1 are at the verge of a major paradigm shift, in which optimal clinical responses will be accomplished by targeting mTORC1-associated metabolic vulnerabilities without inhibiting mTORC1 itself.
©2014 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest: The authors disclose no potential conflicts of interest.
Figures

Similar articles
-
Autophagy-dependent metabolic reprogramming sensitizes TSC2-deficient cells to the antimetabolite 6-aminonicotinamide.Mol Cancer Res. 2014 Jan;12(1):48-57. doi: 10.1158/1541-7786.MCR-13-0258-T. Epub 2013 Dec 2. Mol Cancer Res. 2014. PMID: 24296756 Free PMC article.
-
mTORC1 enhancement of STIM1-mediated store-operated Ca2+ entry constrains tuberous sclerosis complex-related tumor development.Oncogene. 2013 Sep 26;32(39):4702-11. doi: 10.1038/onc.2012.481. Epub 2012 Oct 29. Oncogene. 2013. PMID: 23108404 Free PMC article.
-
Tuberous sclerosis complex inactivation disrupts melanogenesis via mTORC1 activation.J Clin Invest. 2017 Jan 3;127(1):349-364. doi: 10.1172/JCI84262. Epub 2016 Dec 5. J Clin Invest. 2017. PMID: 27918305 Free PMC article.
-
Mourning Dr. Alfred G. Knudson: the two-hit hypothesis, tumor suppressor genes, and the tuberous sclerosis complex.Cancer Sci. 2017 Jan;108(1):5-11. doi: 10.1111/cas.13116. Epub 2017 Jan 23. Cancer Sci. 2017. PMID: 27862655 Free PMC article. Review.
-
Tuberous sclerosis--A model for tumour growth.Semin Cell Dev Biol. 2016 Apr;52:3-11. doi: 10.1016/j.semcdb.2016.01.025. Epub 2016 Jan 24. Semin Cell Dev Biol. 2016. PMID: 26816112 Review.
Cited by
-
Aberrant REDD1-mTORC1 responses to insulin in skeletal muscle from Type 2 diabetics.Am J Physiol Regul Integr Comp Physiol. 2015 Oct 15;309(8):R855-63. doi: 10.1152/ajpregu.00285.2015. Epub 2015 Aug 12. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 26269521 Free PMC article.
-
Multinuclear NMR and MRI Reveal an Early Metabolic Response to mTOR Inhibition in Sarcoma.Cancer Res. 2017 Jun 1;77(11):3113-3120. doi: 10.1158/0008-5472.CAN-16-3310. Epub 2017 Apr 6. Cancer Res. 2017. PMID: 28386017 Free PMC article.
-
Glutamine reliance in cell metabolism.Exp Mol Med. 2020 Sep;52(9):1496-1516. doi: 10.1038/s12276-020-00504-8. Epub 2020 Sep 17. Exp Mol Med. 2020. PMID: 32943735 Free PMC article. Review.
-
Glutamine Metabolism Drives Growth in Advanced Hormone Receptor Positive Breast Cancer.Front Oncol. 2019 Aug 2;9:686. doi: 10.3389/fonc.2019.00686. eCollection 2019. Front Oncol. 2019. PMID: 31428575 Free PMC article.
-
Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies.Am J Med Genet C Semin Med Genet. 2016 Dec;172(4):402-421. doi: 10.1002/ajmg.c.31531. Epub 2016 Nov 18. Am J Med Genet C Semin Med Genet. 2016. PMID: 27860216 Free PMC article. Review.
References
-
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355(13):1345–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

